Section 2 Chapters 7 - 10©
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(#) Indicates That This Book Is Available As Ebook Or E
ADAMS, ELLERY 11.Indigo Dying 6. The Darling Dahlias and Books by the Bay Mystery 12.A Dilly of a Death the Eleven O'Clock 1. A Killer Plot* 13.Dead Man's Bones Lady 2. A Deadly Cliché 14.Bleeding Hearts 7. The Unlucky Clover 3. The Last Word 15.Spanish Dagger 8. The Poinsettia Puzzle 4. Written in Stone* 16.Nightshade 9. The Voodoo Lily 5. Poisoned Prose* 17.Wormwood 6. Lethal Letters* 18.Holly Blues ALEXANDER, TASHA 7. Writing All Wrongs* 19.Mourning Gloria Lady Emily Ashton Charmed Pie Shoppe 20.Cat's Claw 1. And Only to Deceive Mystery 21.Widow's Tears 2. A Poisoned Season* 1. Pies and Prejudice* 22.Death Come Quickly 3. A Fatal Waltz* 2. Peach Pies and Alibis* 23.Bittersweet 4. Tears of Pearl* 3. Pecan Pies and 24.Blood Orange 5. Dangerous to Know* Homicides* 25.The Mystery of the Lost 6. A Crimson Warning* 4. Lemon Pies and Little Cezanne* 7. Death in the Floating White Lies Cottage Tales of Beatrix City* 5. Breach of Crust* Potter 8. Behind the Shattered 1. The Tale of Hill Top Glass* ADDISON, ESME Farm 9. The Counterfeit Enchanted Bay Mystery 2. The Tale of Holly How Heiress* 1. A Spell of Trouble 3. The Tale of Cuckoo 10.The Adventuress Brow Wood 11.A Terrible Beauty ALAN, ISABELLA 4. The Tale of Hawthorn 12.Death in St. Petersburg Amish Quilt Shop House 1. Murder, Simply Stitched 5. The Tale of Briar Bank ALLAN, BARBARA 2. Murder, Plain and 6. The Tale of Applebeck Trash 'n' Treasures Simple Orchard Mystery 3. -

Powerpoint: Furbearer Regulations
2016-2017 and 2017-2018 Furbearer Trapping & Hunting Regulations Oregon Fish and Wildlife Commission June 9, 2016 Derek Broman 1 ODFW Carnivore-Furbearer Coordinator Presentation Overview License and Pelt Price Trends Species and Season Information Regulation Proposals • Sale of Unprotected Mammal Pelts • ODOT Crossing Structures 2 K.Kohl Furtaker License Sales Trends 1980-2015 6,000 Furtakers Furbearer Hunters 5,000 4,000 3,000 # Licenses 2,000 1,000 0 Year 3 New Furtaker License Trends 1996-2015 3,000 Returning Furtakers New Furtakers 2,500 2,000 1,500 # Licenses 1,000 500 0 Year 4 Furtaker Reporting Trend 2004-2014 100 Furtaker Furbearer Hunter 80 60 40 % Reporting % 20 0 Year 5 Average Pelt Prices 2014-2015 & 2015-2016 ─ Beaver ($14 $11) ─ Bobcat ($195 $211) ─ Coyote ($48 $25) AFWA 2015 Report ─ Marten ($28 $20) Average Trapping Expenses ─ Mink ($11 $6) US $1,694 ─ Muskrat ($5 $2) Oregon $1,761 ─ River Otter ($66 $60) ─ Raccoon ($6 $4) Based on Average Harvest ─ Gray Fox ($18 $11) and Prices for All Species: Western Oregon Furtaker: $1,564 ─ Red Fox ($26 $19) Eastern Oregon Furtaker: $2,006 Prices obtained from the Oregon 6 Territorial Council on Furs Species and Seasons 77 D. Budeau Season Recommendations Unprotected Mammals Open Entire Year for: Badger, Coyote, Nutria, Opossum, Porcupine, Skunks, Weasels Included in Harvest Report Protected Mammals No Open Season for: Fisher, Ringtail Cat, Wolverine, Kit Fox, Sea Otter 8 K.Kohl Furbearer Harvest Season Recommendations • No Changes Proposed to Season Dates for Trapping and Hunting Furbearers ─ Beaver Nov. 15 – Mar. 15 ─ Bobcat Dec. -

Marine Mammal Protection Act Complaint
1 Miyoko Sakashita (Cal. Bar # 239639) CENTER FOR BIOLOGICAL DIVERSITY 2 1212 Broadway, Suite 800 Oakland, CA 94612 3 Tel: (510) 844-7108 Fax: (510) 844-7150 4 Email: [email protected] 5 Lalli Venkatakrishnan (Cal. Bar # 323747) 6 CENTER FOR BIOLOGICAL DIVERSITY 1212 Broadway, Suite 800 7 Oakland, CA 94612 Phone: (510) 676-0348 8 Fax: (510) 844-7150 Email: [email protected] 9 10 Attorneys for Plaintiffs 11 UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF CALIFORNIA 12 13 CENTER FOR BIOLOGICAL DIVERSITY, Civ. No. 14 a non-profit organization, and TURTLE ISLAND RESTORATION NETWORK, a COMPLAINT 15 non-profit organization; (Marine Mammal Protection Act, 16 Plaintiffs, 16 U.S.C. § 1361 et seq) 17 v. 18 SCOTT DE LA VEGA1 Acting Secretary, 19 United States Department of Interior; UNITED STATES FISH AND WILDLIFE 20 SERVICE; 21 Defendants. 22 23 24 25 26 27 28 1 Pursuant to Fed. R. Civ. P. 25(d), David Bernhardt’s successor is automatically substituted as a party when Mr. Bernhardt ceases to hold office as Secretary of the U.S. Department of Interior, and any misnomer not affecting the parties’ substantial rights must be disregarded. 1 INTRODUCTION 2 1. This is an action challenging the failure of Defendants Scott de la Vega, Acting 3 Secretary of the Interior, and the United States Fish and Wildlife Service (collectively “the 4 Service”) to comply with their non-discretionary obligations under the Marine Mammal 5 Protection Act (“MMPA”). 16 U.S.C. § 1361, et seq. Specifically, the Service has failed to issue 6 updated stock assessment reports for marine mammals under its jurisdiction—sea otters, polar 7 bears, walruses, and manatees—within the timeframes mandated by the statute. -

Modern Status of Sea Otter Population on the Commander Islands
Modern status of Sea otter population on the Commander Islands Alexander Burdin, Kamchatka branch of Pacific Institute of Geography, RAS, University of Alaska, Fairbanks, ASLC, Sergey Zagrebelny, Commander preserve Commander Population declines Islands (population growing) Background Only 200 nm strait between Commander Island and western Aleutian islands. Dramatic decline of sea otter populations (up to 90% and more reduction), and some other marine mammal species (harbor seal, SSL) across the Aleutian Archipelago and Alaska Peninsula during past several decades. Increasing (13%/year) sea otter population on the Commander Islands. Research Objectives Commander-Aleutian islands c comparisonsomparisons ¾ to better understand ultimate reasons for the decline ¾ to characterize physiology, behavior, and demography of sea otter population near K. ¾ to expand studies of sea otter-kelp forest interactions 2004-2005 winter field work Sea otter mortality, disease, physical conditions monitoring: Carcasses collection / capture : Age/sex composition Necropsy: disease, causes of death, virology, female reproductive tracts, parasites, stomach contents Biosampling 2006 summer work on the Bering Island Totally 33 sea otters were caught using nets and release. 2006 summer work on the Bering Island 27 sea otters (22 females and 5 males) were instrumented with TDR and radio tag Ongoing investigation and future research Goal 1. Ecosystem research: long-term changes in near shore communities under sea otter predation. availability and abundance of sea otter food recourses. analysis of TDR’s data. direct observation on feeding sea otters. scat analysis. Retrospective analysis of sea otter feeding habits on the Commanders. Ongoing investigation and future research Goal II. Monitoring of sea otter population: annual survey (skiff and shore based). -

Natural History of the Southern Sea Otter
Natural History of the Southern Sea Otter C Compiled by Gena Bentall 2017 Description Sea otters are members of the weasel or mustelid family. Like other members of this family, they have very thick fur. In fact, at 850,000 to one million hairs per square inch, they have the thickest fur of any mammal. Their fur consists of two types of hairs, interlocking underfur (which provides insulation) and longer guard hairs (that help water run off the coat). This system traps a layer of air next to their skin so, when fur is well groomed, their skin does not come in contact with sea water. Sea otters are usually dark brown, and some individuals may be progressively lighter colored (grizzled) on the head, neck, chest and forearms due to loss of pigmentation in the guard hairs. Extent of grizzle can be related to age and individual variation. Sea otters are the smallest marine mammal, and with their flipper-shaped hind feet are well adapted to a marine environment. In California adult females weigh 35-60 pounds (16-27 kg); males reach up to 90 pounds (40 kg). Alaskan sea otters are bigger with males weighing as much as 100 pounds (45 kg). Range/Habitat Sea otters once ranged around the North Pacific Rim from Mexico through Alaska, Russia, and Japan. The maritime fur trade of the 1700-1800s brought sea otters to the brink of extinction and fragmented the once continuous population. There are currently 3 subspecies of sea otter, the Northern Sea Otter (Enhydra lutris kenyoni), the Asian, or Russian, Sea Otter (Enhydra lutris lutris) and our Southern, or California, Sea Otter (Enhydra lutris nereis). -
Combining Salience and Network Analyses To
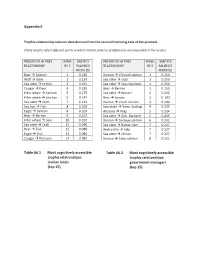
Appendix 6 Trophic-relationship salience data derived from the second freelisting task of the protocol (Table lengths reflect different points at which notable patterns of differences are observable in the results) PREDATOR Æ PREY RANK SMITH’S PREDATOR Æ PREY RANK SMITH’S RELATIONSHIP BY S SALIENCE RELATIONSHIP BY S SALIENCE INDEX (S) INDEX (S) Bear Æ Salmon 1 0.230 Human Æ Chinook salmon 1 0.250 Wolf Æ Deer 2 0.219 Sea otter Æ crab 1 0.250 Sea otter Æ Urchin 3 0.191 Sea otter Æ Sea cucumber 1 0.250 Cougar Æ Deer 4 0.185 Bear Æ Berries 1 0.250 Killer whale Æ Salmon 5 0.175 Sea otter Æ Mussel 2 0.242 Killer whale Æ Sea lion 6 0.147 Bear Æ Leaves 2 0. 242 Sea otter Æ Clam 7 0.133 Human Æ Chum salmon 3 0.240 Sea lion Æ Fish 8 0.109 Sea otter Æ Swm. Scallop 4 0.239 Eagle Æ Salmon 8 0.109 Abalone Æ Kelp 5 0.234 Bear Æ Berries 9 0.107 Sea otter Æ Gnk. Barnacle 5 0.234 Killer whale Æ Seal 10 0.103 Human Æ Sockeye salmon 6 0.231 Sea otter Æ Crab 11 0.090 Sea otter Æ Butter clam 7 0.227 Bear Æ Fish 12 0.088 Red urchin Æ Kelp 7 0.227 Eagle Æ Fish 13 0.086 Sea otter Æ Chiton 7 0.227 Cougar Æ Raccoon 14 0.082 Human Æ Coho salmon 8 0.221 Table A6.1 Most cognitively accessible Table A6.2 Most cognitively accessible trophic relationships: trophic relationships: civilian locals government managers (top 15) (top 15) PREDATOR Æ PREY RANK SMITH’S PREDATOR Æ PREY RANK SMITH’S RELATIONSHIP BY S SALIENCE RELATIONSHIP BY S SALIENCE INDEX (S) INDEX (S) Sea otter Æ Urchin 1 0.264 Killer whale Æ Salmon 1 0.270 Sea otter Æ Clam 2 0.224 Bear Æ Salmon 2 0.267 Wolf Æ Deer 3 0.198 Wolf Æ Deer 3 0.236 Bear Æ Salmon 4 0.184 Cougar Æ Deer 4 0.189 Cougar Æ Deer 5 0.180 Killer whale Æ Sea lion 5 0.138 Bear Æ Berries 6 0.163 Sea otter Æ Urchin 6 0.134 Killer whale Æ S. -

PUP 681: a SEA OTTER RESCUE STORY Curriculum and Storytime Guide
Pup 681: Storytime andA StoryCurriculumtime Guideand Curriculum Guide for 1 Pup 681: A Sea Otter Rescue Story by Jean Reidy, illustrated by Ashley Crowley PUP 681: A SEA OTTER RESCUE STORY ©2019 by Jean Reidy; Art by Ashely Crowley; Henry Holt and Company Praise for Pup 681 “Unabashedly adorable” ~ Kirkus Reviews, starred review “...otter enthusiasts will likely enjoy the tender relationship between 681 and her attentive caregiver.” ~ Publishers Weekly Learning activities align with CCSS (Common Core State Standards) and NGSS (Next Generation Science Standards) where applicable. Guide created by Natalie Lorenzi (www.nataliediaslorenzi.com) Artwork ©Ashely Crowley Pup 681: Storytime and Curriculum Guide 2 Story Summary Washed ashore alone, a tiny sea otter pup needs help! Soon, a rescuer is there to take her in and keep her warm and fed. The pup faces challenges in her new life without her sea otter family. But with the love and care of her rescuer, she flourishes in her new home. Inspired by a true story, this is a heartwarming and hopeful tale about family and love. About the Author Jean Reidy’s fun, lively and award-winning picture books have earned their spots as favorites among readers and listeners of all ages and from all over the world. She is a frequent presenter on writing and reading and at schools and storytimes across the country—in person and virtually. Jean is a member of the Society of Children’s Book Writers and Illustrators, the National Council of Teachers of English, the Colorado Council International Reading Association and she has served on the board of Reach Out and Read Colorado. -

Fact Sheet Regulations for Marine Mammal Parts Beach Found by Non-Natives
FACT SHEET REGULATIONS FOR MARINE MAMMAL PARTS BEACH FOUND BY NON-NATIVES January 2001 Fish and Wildlife Service ! U.S. Department of the Interior The U.S. Fish & Wildlife Service manages sea otters, polar bears and walrus in Alaska. This fact sheet addresses often asked questions about beach found marine mammal parts collected by Non- Natives. Similar Fact Sheets addressing Alaskan Natives and marine mammals are also available. For answers to specific questions please contact one of the offices listed on the back of this sheet. WHO MAY COLLECT BEACH FOUND PARTS? Federal regulations allow the collection of parts by Non-Natives (and Natives) from some dead marine mammals found on the beach or land within 1/4 mile of the ocean (including bays and estuaries), depending on land ownership. WHERE CAN BEACH FOUND PARTS BE COLLECTED? Regulations vary depending on land ownership. It is the collector=s responsibility to know whose lands they are visiting. Collectors should check for additional regulations established by individual landowners (Federal, State, or private) before removing any resource. Collection of all animal parts (including marine mammals) is prohibited on National Park Service lands. WHAT PARTS MAY BE COLLECTED? Skulls, bones, teeth or ivory from beach found sea otter, polar bear and walrus may be collected. The skins, meat and organs from these animals may not be collected. Animal parts (including marine mammals) of an archeological or paleontological origin may not be collected from Federal or State lands. WHAT ABOUT OTHER MARINE MAMMALS? The National Marine Fisheries Service (NMFS) has responsibility for managing whales, seals, sea lions, dolphins, and porpoises. -

Introduction to the Otter Program (Pilot)
Introduction to the Otter Program (pilot) The Otter Raft A Section of the Scouting Group aimed at youngsters aged from 5 to 7 years. The Scouter- in-charge of the Raft will be the Otter Leader and he/she will be assisted by Assistant Otter Leaders. Parents may also be called upon to assist. The maximum number of Otters permitted in one Pack is 32 (4 dens of 8 Otters each). The Den The Raft will be divided into a number of Dens, each Den to consist of a maximum of 8 Otters but preferably not more than 6 Otters. The Otter in charge of a Den is called a Den Leader and he/she is assisted by an Assistant Den Leader. Where there is an outstanding Otter who is in the later stages of the training scheme then he/she may be appointed as Raft Leader. This is a similar position to a Senior Sixer in the Timberwolf Pack. The Raft Leader will assist the Otter Leader. The Den will normally be the working unit. The Group The Group will consist of one or more Sections but will not be thought to be complete until all Sections possible are fully operating. A complete Scouting Group will consist of: An Otter Raft; A Timberwolf Pack; A Pathfinder Troop; A Rover Crew. The Scouting Group will be in the charge of a Group Scouter and each Section of the Group will have a Section Leader with Assistants. The Group Council This will consist of all the registered Scouters in the Group and this Council will, under the guidance of the Group Scouter, deal with all matters affecting the training of the Group. -

Sea Otter Critical Habitat in Southwest Alaska
Sea Otter Critical Habitat in Southwest Alaska On October 8, 2009, the U.S. Fish and Wildlife Service (Service) finalized designation of 15,164 km2 (5,855 mi2) of critical habitat for the threatened northern sea otter in southwest Alaska. This designation is essentially the same areas we proposed on December 16, 2008 (73 FR 76454). The final rule and final economic analysis can be viewed at http://alaska.fws.gov/fisheries/mmm/ seaotters/criticalhabitat.htm. What is “critical habitat?” “Critical habitat” is a term in the Endangered Species Act (ESA) that identifies geographic areas that contain the specific habitat elements essential for the conservation of a threatened or endangered species, and which may require special management considerations or protection. Federal Sea otters are often found in shallow, nearshore marine waters. agencies that undertake, fund or permit activities that may affect critical habitat habitat are required to consult with the When the Service requested public are required to consult with the Service Service to ensure such actions do not comments on the proposed listing, we to ensure such actions do not adversely adversely modify or destroy critical also requested information regarding modify or destroy designated critical habitat. The designation of critical features and specific areas that might habitat. habitat does not affect land ownership have helped us designate critical or establish a refuge, wilderness, habitat. The Service did not receive Where is the critical habitat located? reserve, preserve, or other conservation sufficient information at that time The southwest Alaska distinct area. It does not allow government or to designate critical habitat. -

2018-2019 and 2019-2020 Furbearer Trapping and Hunting Regulations
2018-2019 and 2019-2020 Furbearer Trapping and Hunting Regulations Oregon Fish and Wildlife Commission June 7, 2018 Derek Broman 1 ODFW Carnivore-Furbearer Coordinator Presentation Overview • License and Pelt Price Trends • Species and Season Information • General Regulation Proposals • Program Notes 2 K.Kohl Furtaker License Sales Trends 1987-2017 3,500 Furtakers Furbearer Hunters 3,000 2,500 2,000 1,500 # Licenses # 1,000 500 0 Year 3 New Furtaker License Trends 1997-2017 3,000 Returning Furtakers New Furtakers 2,500 2,000 1,500 # Licenses # 1,000 500 0 Year 4 Furtaker Reporting Trend 2007-2017 100 Furtakers Furbearer Hunter 80 60 40 Time and Late) Time 20 Reporting - (On % 0 Year 5 Furtaker Reporting Trend 2007-2017 On-Time Reporting Furtakers Furbearer Hunters Average 100 80 60 40 Time Reporting Time - 20 On % 0 Year 6 $800 Average $600 Annual $400 E Bobcat Otter Pelt $200 W Bobcat $0 Prices 2010 2011 2012 2013 2014 2015 2016 2017 $80 AFWA $60 2015 Marten $40 Mink Report- $20 Red Fox Average Gray Fox $0 Trapping 2010 2011 2012 2013 2014 2015 2016 2017 Expenses: $30 US $20 Beaver $1,694 Raccoon $10 Oregon Muskrat $1,761 $0 7 2010 2011 2012 2013 2014 2015 2016 2017 Species and Seasons 78 D. Budeau Season Recommendations Unprotected Mammals Open Entire Year for: Badger, Coyote, Nutria, Opossum, Porcupine, Skunks, Weasels Most Included in Harvest Report Protected Mammals No Open Season for: Fisher, Ringtail, Wolverine, Kit Fox, Sea Otter 9 K.Kohl Furbearer Harvest Season Recommendations • No Proposed Changes to Season Dates for most Furbearer Trapping and Hunting ─ Beaver Nov. -

DRAGON Magazine Is Available at Hobby
DRAGON 1 Publisher: Mike Cook Editor-in-Chief: Kim Mohan One little word Editorial staff: Roger Raupp Patrick Lucien Price Those of us who work with words are Mary Kirchoff seldom surprised by instances where one Vol. IX, No. 3 August 1984 Roger Moore Subscriptions: Mellody Knull little word makes a lot of difference. Thats SPECIAL ATTRACTION Contributing Editors: Ed Greenwood the way it is with words, we say knowingly. Katherine Kerr But we got a letter the other day that was Ken Rolston surprising. ELEFANT HUNT. .45 Advertising Sales Administrator: It came from Eric Dott, president of Safari, so good: The latest game Mary Parkinson Monarch Avalon Industries, Inc., and the from Tom Whams imagination This issues contributing artists: body of the letter goes like this: Please be James Holloway Keith Parkinson advised that from this date forward, every Jeff Butler Brian Born reference to The Avalon Hill Game Com- OTHER FEATURES Roger Raupp Kurt Erichsen pany in your publication MUST be stated Tom Wham Dave Trampier as The Avalon Hill Game Company and Falling damage: a matter of gravity Mark Nelson Larry Elmore nothing else. It is important that the defi- nite article The precede the words Avalon Physics and falling damage. .12 DRAGON® Magazine (ISSN 0279-6848) is published monthly for a subscription price of $24 Hill Game Company. Any deviation from The argument in favor of velocity per year by Dragon Publishing, a division of the full phrase The Avalon Hill Game TSR, Inc. The mailing address of Dragon Company or any use of the word Avalon Scientific facts behind the system.