CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ahmedabad Municipal Corporation Councillor List (Term 2021-2026)
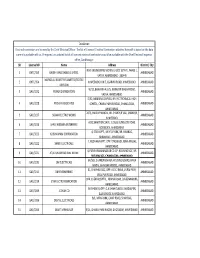
Ahmedabad Municipal Corporation Councillor List (term 2021-2026) Ward No. Sr. Mu. Councillor Address Mobile No. Name No. 1 1-Gota ARATIBEN KAMLESHBHAI CHAVDA 266, SHIVNAGAR (SHIV PARK) , 7990933048 VASANTNAGAR TOWNSHIP, GOTA, AHMEDABAD‐380060 2 PARULBEN ARVINDBHAI PATEL 291/1, PATEL VAS, GOTA VILLAGE, 7819870501 AHMEDABAD‐382481 3 KETANKUMAR BABULAL PATEL B‐14, DEV BHUMI APPARTMENT, 9924136339 SATTADHAR CROSS ROAD, SOLA ROAD, GHATLODIA, AHMEDABAD‐380061 4 AJAY SHAMBHUBHAI DESAI 15, SARASVATINAGAR, OPP. JANTA 9825020193 NAGAR, GHATLODIA, AHMEDABAD‐ 380061 5 2-Chandlodia RAJESHRIBEN BHAVESHBHAI PATEL H/14, SHAYONA CITY PART‐4, NR. R.C. 9687250254, 8487832057 TECHNICAL ROAD, CHANDLODIA‐ GHATLODIA, AHMDABAD‐380061 6 RAJESHWARIBEN RAMESHKUMAR 54, VINAYAK PARK, NR. TIRUPATI 7819870503, PANCHAL SCHOOL, CHANDLODIA, AHMEDABAD‐ 9327909986 382481 7 HIRABHAI VALABHAI PARMAR 2, PICKERS KARKHANA ,NR. 9106598270, CHAMUDNAGAR,CHANDLODIYA,AHME 9913424915 DABAD‐382481 8 BHARATBHAI KESHAVLAL PATEL A‐46, UMABHAVANI SOCIETY, TRAGAD 7819870505 ROAD, TRAGAD GAM, AHMEDABAD‐ 382470 9 3- PRATIMA BHANUPRASAD SAXENA BUNGLOW NO. 320/1900, Vacant due to Chandkheda SUBHASNAGAR, GUJ. HO.BOARD, resignation of Muni. CHANDKHEDA, AHMEDABAD‐382424 Councillor 10 RAJSHRI VIJAYKUMAR KESARI 2,SHYAM BANGLOWS‐1,I.O.C. ROAD, 7567300538 CHANDKHEDA, AHEMDABAD‐382424 11 RAKESHKUMAR ARVINDLAL 20, AUTAMNAGAR SOC., NR. D CABIN 9898142523 BRAHMBHATT FATAK, D CABIN SABARMATI, AHMEDABAD‐380019 12 ARUNSINGH RAMNYANSINGH A‐27,GOPAL NAGAR , CHANDKHEDA, 9328784511 RAJPUT AHEMDABAD‐382424 E:\BOARDDATA\2021‐2026\WEBSITE UPDATE INFORMATION\MUNICIPAL COUNCILLOR LIST IN ENGLISH 2021‐2026 TERM.DOC [ 1 ] Ahmedabad Municipal Corporation Councillor List (term 2021-2026) Ward No. Sr. Mu. Councillor Address Mobile No. Name No. 13 4-Sabarmati ANJUBEN ALPESHKUMAR SHAH C/O. BABULAL JAVANMAL SHAH , 88/A 079- 27500176, SHASHVAT MAHALAXMI SOCIETY, RAMNAGAR, SABARMATI, 9023481708 AHMEDABAD‐380005 14 HIRAL BHARATBHAI BHAVSAR C‐202, SANGATH‐2, NR. -

Ahmedabad, Gujarat
SHARAN VENTURE LLP Form 1, Form 1(A) and Proposed ToR for Residential Project Moje: Tragad, Taluka: Ghatlodiya, District: Ahmedabad, Gujarat. DECEMBER 2016 PROPOSED RESIDENTIAL PROJECT AT SHARAN VENTURE LLP TRAGAD, AHMEDABAD,GUJARAT SCOPING – FORM 1 CONTENTS 1 SCOPING ................................................................................................... 4 1.1 FORM – 1 ............................................................................................... 4 1.2 FORM – 1(A) ......................................................................................... 16 PAGE 2 PROPOSED RESIDENTIAL PROJECT AT SHARAN VENTURE LLP TRAGAD, AHMEDABAD,GUJARAT SCOPING – FORM 1 LIST OF ANNEXURES Annexure 1: Toposheet .................................................................................................................................... 27 Annexure 2: Soil Analysis Report ...................................................................................................................... 28 Annexure 3: Source of Water Supply, Sewer line connection and Solid Waste Management Permission Letter ...... 29 Annexure 4: NA documents, Land Possession document (7/12), TP scheme map and location of project on it, Zoning Certificate, Agreement between land owner and developer ..................................................................... 30 Annexure 5: Satellite Map................................................................................................................................. 35 Annexure 6: Land Use Map.............................................................................................................................. -

Notification Under Section 144 of Code of Criminal Procedure, 1973 (No
-:: Notification under Section 144 of Code of Criminal Procedure, 1973 (No. 2 of 1974), Section 33(L) of Gujarat Police Act, 1951 and Section 34 of National Disaster Management::- No: DC/MG/CORONA/Notification/S.R. 50/2020 Office of Collector and District Magistrate Near Subhashbridge, Ashram Road, Ahmedabad Date: 19/04/2020 Referred: - 1. MHA Letter No. 40-3/2020-DM-1(A) dated 15/04/2020 2. GOG Home Department Notification No. GG/23/2020/Part1/KAV/102020/482 dated 14/04/2020 3. This office Notification No. DC/MG/CORONA/Notification/S.R.48/2020 dated 15/04/2020 4. GOG IMD Letter No. MIS-112020-GOI-10-G dated 15/04/2020 5. This office Order No. EBC/EST/CORONA/Lockdon-2/Committee/2020 dated 17.04.2020 6. GOI IMD Letter No. MIS/112020-GOI-10-G dated 19/04/2020. -::NOTIFICATION::- Currently World Health Organization has declared CORONA-19 as epidemic globally. Multiple COVID-10 cases are recorded in India too, due to which the Central Government had declared lockdown till 14.04.2020. To stop the speedy spreading of COVID-19 infection in crowded public and personal place the notification was published. The central government has extended the lockdown till 03.05.2020. So, the GOG has notified to stop all the services from 15.04.2020 (00.00 Hours) to 03.05.2020 (24:00 Hours) except essential services. The GOI has published detailed guidelines to stop spreading COVID-19 wherein in the para-3 of guidance it is mentioned that the certain relaxation will be given to minimize public inconvenience. -
Portal Electrical Contactor List.Xlsx
Disclaimer: Electrical contractors are licenced by the Chief Electrical Officer. The list of Licenced Electrical Contractors attached herewith is based on the data currently available with us. If required, an updated list of all licenced electrical contractors would be available with the Chief Electrical Inspector office, Gandhinagar. SR License NO Name Address District / City R.M. ENGINEERING WORKS 3, GIDC ESTATE, PHASE 1, 1 GHTC/359 RAJESH MALLOABLES LIMITED. AHMEDABAD VATVA, AHMEDABAD - 382445 MAFATLAL INDUSTRIES LIMITED (TEXTILE 2 GHTC/354 AHMEDABAD UNIT, ASARWA ROAD, AHMEDABAD. AHMEDABAD DIVISION) W/12, BHAVANA FLATS, NARAYAN NAGAR ROAD, 3 GAC/3232 POWER DISTRIBUTORS AHMEDABAD VASNA, AHMEDABAD. D/92, NIRMAN COMPLEX, NR. R.C.TRCHNICAL HIGH 4 GAC/3228 PUSHYA ASSOCIATES SCHOOL, CHANAKYAPURI ROAD, CHANDLODIA, AHMEDABAD AHMEDABAD. 2675, HAIDAR MANZIL, NR. SHAHPUR VAD, SHAHPUR, 5 GAC/3227 SIGMA ELECTRIC WORKS AHMEDABAD AHMEDBAD. A/51,SHAKTI ENCLAVE, JUDGES BUNGLOW ROAD, 6 GAC/3226 SHREE KRISHNA ENTERPRISE AHMEDABAD BODEKDEV, AHMEDABAD. 4, STAR APPT., MEHTA PARK, NR. HIRABAG, 7 GAC/3225 ADISHVARAM CORPORATION AHMEDABAD AMBAWADI, AHMEDABAD. 7, NILDHARA APPT, OPP. VYASWADI, NAVA WADAJ, 8 GAC/3222 SHREE ELECTRICALS AHMEDABAD AHMEDABAD. 4, PARIBHRAHAMNAGAR CO.OP. HOUSING SOC, NR. 9 GAC/3221 ATULKUMAR RASIKLAL NAYAK AHMEDABAD RUTURAJ SOC, CHANDLODIA, AHMEDABAD. 64/583, CHANDRABHAGA HOUSING BOARD, NAVA 10 GAC/3220 OM ELECTRICALS AHMEDABAD WADAJ, BHAVSAR HOSTEL, AHMEDABAD. 51, SHAIWALI SOC, OPP. A.D.C. BANK, JIVRAJ PARK, 11 GAC/3215 OM ENGINEERING AHMEDABAD VEJALPUR ROAD, AHMEDABAD. OPP. CHEPI HOSPITAL, PIRANA ROAD, BAHERAMPURA, 12 GAC/3214 STAR ELCTRO FEBRICATION AHMEDABAD AHMEDABAD. AVISHKAR II, OPP. G.A.SHAH CLASSES, MADALPUR, 13 GAC/3209 JCSHAH CO AHMEDABAD ELLIS BRIDGE,AHMEDABAD. -

Shabdalpura (44) Family List
Shabdalpura (44) Family List SN Name Address Mobile No D-1, Sunrise Park, Opp. New Chaitanya Nagar, Nr. 1 Patel Amaratbhai Mangaldas Muni. School, Shahibaug, Ahmedabad. 9427026221 K-15, Shayona City, Part-4, K-I-S, Shayona City, 2 Patel Amrutbhai Prahladbhai Ghatlodiya, Ahmedabad. 9824545440 C-4, Shantidayal Apartment, Gurukul Road, 3 Patel Arvindbhai Bechardas Memnagar, Ahmedabad. 9925463092 B-4, Ghanshyamnagar Society, Nr. Raghunath Hindi 4 Patel Arvindbhai Manilal Highschool, Bapunagar, Ahmedabad. 9427320926 25, Saibaba Society, Opp. Somnath Mahadev, 5 Patel Babulal Ranchhoddas Meghaninagar, Asarva, Ahmedabad. 9724411608 K-11, Shayona City, Part-4, R.C.Tech Road, 6 Patel Babubhai Shankardas Ghatlodiya, Ahmedabad. 9426049183 C-4, Umiya Apartment, Part-1, Nr. Kankeshvari 7 Patel Bharatbhai Ambalal Society, Krushnanagar, Ahmedabad. 9427800935 B-10, Nusinh Park Society, Vijay Cross Road, 8 Patel Bipinchandra Keshavlal Naranpura, Ahmedabad. 9898107678 B-103, Sopan Residency, Opp. Shikhar Apartment, 9 Patel Bharatbhai Bachudas Nikol-Naroda Road, New Naroda, Ahmedabad. 9426557829 B-14, Manmohan Society, B/H Raghunath 10 Patel Bharatbhai Babaulal Highschool, Bapunagar, Ahmedabad. 9974391402 B-26, Puja Apartment, B/H Kailash Flat, 11 Patel Bhikhabhai Gandalal Mahaveernagar Road, Hirawadi, Ahmedabad. 9427042394 B-403, Arjun Greens, B/H Nilkanth Mahadev, 12 Patel Bhupendrabhai Shankarlal Naranpura, Ahmedabad. 9824782164 1, Varniraj Society, Opp. Nandigram Society Part-1, 13 Patel Dashrathbhai Mangaldas Sanghavi Railway Crossing, Nr. Vedhschool, 9426708280 Narayanpura, Ahmedabad. B-8, Umiya Apartment, nr. Kankeshvari Society, 14 Patel Dilipbhai Ambalal Sardar Chowk, Nr. Ankur School, Krushnanagar, 9723278411 Ahmedabad. 17, Kamal Society, Nr. Swaminarayan Baug, 15 Patel Gangarambhai Shivarambhai Memnagar, Ahmedabad. 27417515 B-402, Shantiniketan Flat, Nikol Road, Opp. Raspan 16 Patel Girishbhai Ramanlal Party Plot, New Naroda, Ahmedabad. -

Life Membership 2011-18 Dec Telephone
LIFE MEMBERSHIP 2011-18 DEC TELEPHONE . Sl.No Name Designation ADDRESS NO SABZAZRE_NASEEBA-17,Muslim 1 A A MUNSHI HD Pharmasist soc. Navrangpura, AHMEDABAD 9979148439 380009. Gineshwar part - I Nr Kanti part 2 A B PANT EE(D) society,Ghatlodia Ahmedabad- 27604204 380061 B-32, Orchid park nr Shailby Hospital 3 A C Bajaj Mgr(Logistic) 9904981023 opp. Karnavati c;lub Satelite , A-22,Park Avenue New cg 4 A C Barua Dy.SE(P) 9427336696 roadChandkheda Ahmedabad-380005 22,Somvil Bunglows,Bhaikaka Nagar 5 A C Saini SE(P) Thaltej Ahmedabad-55 B-302.Chinubhai Tower Satelite 6 A D PATEL SE(P) 9428563893 Memnagar AHMEDABAD-52. 27,Konark Society Sabarmati 7 A D VAID DySE(E) 9898218428 Ahmedabad -380019 A-9 AL-Ashurfi Society,B/H Haider 8 A G SHAIKH AEE(P) Nagar, JUHAPAURA, Sarkhej Road 9428330591 AHMEDABAD 380055 B-27, Shardakrupa Society, B/H 9 A H Naik Dy. SE(P) Janatanagar Chandkheda, 27516085 GANDHINAGAR-382424. 66/7 Madini Chamber ,Mahakali 10 A I Shaikh AE(M) Temple Dudeshwar Shahibaug 9824591030 Ahmedabad 8 Sindhu Mahal soc. Ashram road Old 11 A J Sharma DM(HR) 9428008152 Vadaj Ahmedabad 380013. D-303 Aditya residency Motera 12 A K Dhawan GM(Res) 9428332121 Ahmedabad 380005. H-6,Karnavati Soc.GHB Chandkheda 13 A K GAHLAUT GM(P) 23296926 Ahmedabad-382424 Flat no 1001 Sangath Diomond 14 A K Gupta Exe.Director Tower nr PVR cinema Motera 9712922825 Ahmedabad 380005. 2nd floor Rituraj Apartment op Rupal 15 A K Gupta DGM(MM) flats nr Xavier Loyla school 9426612638 Ahmedabad B-77,RJESHWARI 09428330135- 16 A K MEHTA EE(M) SOCIETY,PO,TRAGAD,IOC ROAD 27508082 CHANDKHEDA AHMEDABAD-382470. -

STATE District CITY ADDRESS OFFSITE/ONSITE GUJARAT
STATE District CITY ADDRESS OFFSITE/ONSITE AXIS BANK ATM 1 MAHAVIR SMRUTI SOCIETY C P NAGAR GUJARAT AHMEDABAD GHATLODIYA OFFSITE CHAR RASTA GHATLODIA AHMEDABAD AXIS BANK ATM 1/2 , AMI APPARTMENT , OPP.NARANPURA GUJARAT AHMEDABAD AHMEDABAD TELEPHONE EXCHANGE , NARANPURA-AHMEDABAD - OFFSITE 380013, GUJARAT AXIS BANK ATM 1056 INDIRA NAGAR PART 2 LAMBHA GUJARAT AHMEDABAD AHMEDABAD OFFSITE AHMEDABAD 382405 AXIS BANK ATM 12,ROYAL NAWAB, CHANDOLA TALAW GUJARAT AHMEDABAD AHMEDABAD ROAD, SHAH E ALAM, DANILIMDA,AHMEDABAD - OFFSITE 380028,STATE- GUJARAT AXIS BANK ATM 13/A , ISHWARCHARAN GUJARAT AHMEDABAD AHMEDABAD COMPLEX,OPP.RIDDHI TOWER,JODHP RGAM SHAK OFFSITE MARKET,SATELLITE,AHMEDABAD-380015 AXIS BANK ATM 16- SHIV DARSHANDHAM SOCIETY, OPP GUJARAT AHMEDABAD AHMEDABAD SUVAS ORAM, NR SP RING ROAD, ODHAV, AHMEDABAD OFFSITE 382415, GUJARAT AXIS BANK ATM 16, GOKUL DHAM SOCIETY, NARODA GUJARAT AHMEDABAD NARODA KATHWADA RD, NR. MURLIDHAR SOCIETY, NARODA, OFFSITE AHMEDABAD-382325, AHMEDABAD, GUJARAT, 382325 AXIS BANK ATM 17 2 UMANG FLAT OPP ANAND FLAT POLICE GUJARAT AHMEDABAD AHMEDABAD CHOWKEY NR LAL BAHADUR SHASHTRI STEDIEUM OFFSITE BAPUNAGAR AHMEDABAD 380024 AXIS BANK ATM 1747,ROHILA WAD,NR JAY SHANKAR SUNDRI GUJARAT AHMEDABAD AHMEDABAD OFFSITE HALL,RAIKHAD,AHMEDABAD-380001 AXIS BANK ATM 19,GOPLANI MARKET,NARODA PATIYA GUJARAT AHMEDABAD AHMEDABAD OFFSITE CROSS ROAD, NARODA, AHMEDABAD. 382330 AXIS BANK ATM 1-GANESHWARI SOCIETY, NR GUJARAT AHMEDABAD AHMEDABAD OFFSITE CHHAGANBHAI'S WADI, ADINATHNAGAR, ODHAV 382415 AXIS BANK ATM 2- EWARD RAJVIR CIRCLE, -

Unclaimed Dividend 2013-14 As on 19-09-15
First Middle Last Father/H Father/H Father/H Address Country State District PINCode Folio Number of Investm Amount Proposed Name Name Name usband usband usband Securities ent Type Due(in Date of First Middle Last Rs.) transfer to Name Name Name IEPF (DD- MON-YYYY) BALVANTSINGH RATHOD DALPATSINGH B NO 1 SWAMINARAYAN COLONY C T,OPP VISHNU PETROL PUMP,,AHMEDABAD INDIA GUJARAT AHMEDABAD 0010307 Amount for150.00 unclaimed28-OCT-2021 and unpaid dividend BIPIN SOMAIYA PRABHUDAS 2 - GYAN JIVAN SOCIETY,,BRAHAM SAMAJ, RAIYA ROAD,,OPP. BHAVANI KRUPA,,RAJKOT INDIA GUJARAT RAJKOT 0010380 Amount for150.00 unclaimed28-OCT-2021 and unpaid dividend DHERMENDRAM PATEL MAFATLAL AT POST SAKRA,TALUKA HARIJ,,MEHSANA INDIA GUJARAT MEHSANA 0010475 Amount for150.00 unclaimed28-OCT-2021 and unpaid dividend GIRDHARBHAI SACHANIA RANCHHODBHAI 40 VAIBHAVPARK SOC,NR ATMAMANGAL HALL GHODASAR,,AHMEDABAD INDIA GUJARAT AHMEDABAD 0010541 Amount for150.00 unclaimed28-OCT-2021 and unpaid dividend HIRABEN D PATEL DHERMENDRA AT POST SAKRA,TALUKA HARIJ,,MEHSANA INDIA GUJARAT MEHSANA 0010620 Amount for150.00 unclaimed28-OCT-2021 and unpaid dividend KAMLA DEVI RAM AUTAR 52/570, PRATAP NAGAR,SANGANER,,JAIPUR INDIA RAJASTHANJAIPUR 0010768 Amount for150.00 unclaimed28-OCT-2021 and unpaid dividend NATWARBHAI PATEL ISHWARBHAI MAMTA SOCIETY NR WATER TANK,VATVA,,AHMEDABAD INDIA GUJARAT AHMEDABAD 0011039 Amount for150.00 unclaimed28-OCT-2021 and unpaid dividend PRADEEP KUMAR GAUTAM PRAVESHKUMAR P-III/14, SINGH DWAR COLONY,(WORLD BANK), GURUKUL KANGRI,HARDWAR,U P INDIA UTTAR PRADESHHARDWAR -

SN Name Address Mobile No PALADI VYAS (94) Family List
PALADI VYAS (94) Family List SN Name Address Mobile No G-2, KEDAR FLAT, SAIBABA SOCIETY NI SAME, RAMESVAR, 1 AMARIBHAI GOKALDAS PATEL MEGHANINAGAR, AHMEDABAD-16 9376606506 18, NAYAN RATN APARTMENT, NAGARPALIKA NI SAME, GAYTRI MANDIR 2 AMRUTBHAI KALIDAS PATEL ROAD, RANIP AHMEDABAD-382480 9428804236 M-2, KRUPA RESIDENCY, SVSTIK SCHOOL NI BAJUMA MOTERA STEDIYAM 3 AMRUTBHAI JOITARAM PATEL ROAD, MOTERA AHMEDABAD-24 9925812720 19/218, NETAJINAGAR AMBAJI MATANA MANDIR PASE, MEGHANINAGAR 4 AMRUTBHAI NARANDAS PATEL AHMEDABAD-16 9924317520 296, BHARGAV TENAMENT, BHARGAV ROAD, KUBERNAGAR, AHMEDABAD- 5 AMRUTBHAI SOMABHAI PATEL 382340 9979577823 B-402, SUDAMA APARTMENT, VASTRAL ROAD, RABARI COLONY CHAR 6 ARVINDBHAI NARAYANDAS PATEL RASTA PASE, AMRAIVADI AHMEDABAD 9979771775 A-302, GOKUL APARTMENT, SARVODAY SCHOOL NI NAJIK, VAGHESVARI 7 ARVINDBHAI SOMABHAI PATEL ROAD, K.K.NAGAR, GHATLODIYA AHMEDABAD-380061 9879986163 K-16, VISVAS FLAT, RANGSAGAR SOCIETY PASE, SARDAR CHOK, 8 AHOKBHAI AMBALAL PATEL KRUSHNA, AHMEDABAD-382346 9904788005 C-4, YAMUNA SOCIETY, RAMAJI MANDIR NI BAJUMA, BHARGAV ROAD, 9 AMBALAL NAGARDAS PATEL KUBERNAGAR, AHMEDABAD-382340 9825056945 66, UMIYANAGAR SOCIETY, RAGHUNATH HINDI HIGHSCOOL NI BAJUMA 10 BABUBHAI SOMDAS PATEL L.B.SHASTRI ROAD,BAPUNAGAR AHMEDABAD-380024 8000223487 30, NAVDIP PELES, SHIPA MEDICAL NI PACHHAL, HIRAVADI, 11 BHARATBHAI KANJIBHAI PATEL MAHAVIRNAGAR ROAD, AHMEDABAD-382345 9428115367 5, NARNARAYAN EVANYU, BHAGYODAY TENAMENT NI PACHHAL, NARODA 12 BHARATBHAI NARAYANDAS PATEL BETHAK TRAN RASTA, NARODA, AHMEDABAD 9904902728 -
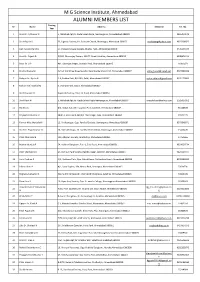
ALUMNI MEMBERS LIST Passing Re Name Address E Mail ID Tel
M G Science Institute, Ahmedabad ALUMNI MEMBERS LIST Passing Re Name Address E Mail ID Tel. No. Year 1 Shah Dr. Ajitkumar P. 5, Abhishek Apt;Nr. Dada Saheb Pagla, Navrangpura, Ahmeddabad-380009 9825019572 2 Shah Rajesh V. 33, Uganda Society, Nr. Subhash Chawk, Memnagar, Ahmeabad-380052 [email protected] 9825008925 3 Dani Sureshchandra. 17, Dungarshinagar Society, Bhatta, Paldi, Ahmedabad-380007 9426543344 4 Shah Dr. Rajesh D. D/502, Dhananjay Towers, 100 FT. Road, Satellite, Ahmedabad-380015 9898874111 5 Patel Dr. V.P. 401, Saraswati Nagar, Himatlal Park, Ahmedabad-380015 26302871 6 Shukla Alpana M. Ashish' 14, Shree Nivas Society, New Sharda Mandir Rd. Ahmedabad-380007 [email protected] 9327000264 7 Rafique Dr. Aysha N. 5-A, Prabha Park, B/H NID, Paldi, Ahmedabad-380007 [email protected] 9376179846 8 Mehta Prof. Rasiklal M 9, Vishram Park, Vasna, Ahmedabad-380007 9 Shah Paulomi H Gopinath Society, Drive-in-Road, Ahmedabad-380054 10 Shah Karvi N 5, Abhishek Apt; Nr. Dada Saheb Pagla,Navrangpura, Ahmedabad-380007 [email protected] 9925010951 11 Rai Hita S. B/2, Adesh Apt; B/h Hasubhai Park, Satellite, Ahmedabad-380007 55120678 12 Prajapati Babubhai B 38/413, Greenpark Apt;B/h. Parasnagar, Sola, Ahmedabad-380062 27412175 13 Parmar Miss Manjula M 12, Vinabanagar, Opp. Panchsil Society, Usmanpura, Ahmedabad-380007 9375948071 14 Shah Dr. Piyushkumar N 28, Shrinath Krupa, Nr. Sarddar Patel School, Maninagar, Ahmedabad-380007 25463470 15 Patel Chimanlal R 123, Alkapuri Society, Ghatlodiya, Ahmedabad-380061 27454665 16 Madan Manjula P 54, Vaibhav Bunglows, Part-1, Sola Road, Ahmedabad-380061 9824050734 17 Patel Shantaben K 24, Jodhpur Park Society, Ramdevnagar, Satellite, Ahmedabad-380015 9825253977 18 Joshi Pratima G A/3, Rainbow Flats, Opp. -

Ele Contract List New.Xlsx
Disclaimer: Electrical contractors are licenced by the Chief Electrical Officer. The list of Licenced Electrical Contractors attached herewith is based on the data currently available with us. If required, an updated list of all licenced electrical contractors would be available with the Chief Electrical Inspector office, Gandhinagar. License NO Party Name Address District SPVR_PERMIT_NO From Date To Date 12/4 VIJAY ESTATE GAC/3646 SHRI UMIYA ELECTRICALS OPP.BHIKSUKGRUH ODHAV AHMEDABAD GAS/E-17379 01-01-2014 31/12/2016 A''BAD 353,SAMIRNAGAR MAHESHWARINAGAR,NR.BHIKS G/AHD/C-2370 RAJAN ENTERPRISE AHMEDABAD G/GS-E-001743-DEE-2010 01-01-2014 31/12/2016 HUK GRUH,ODHAV AV,AHMEDABAD 19,JANTA MARKET G/AHD/C-2712 D FIVE ENGINEERING SOCIETY,NR.VEJALPUR BUS AHMEDABAD G/AS/E-5701 01-01-2014 31/12/2016 STAND,VEJALPUR,AHMEDABAD 15,ARTI G/AHD/C-2687 JAY AMBE ELECTRICALS TENAMENT,NR.SWAMINARAYAN AHMEDABAD G/GS-E-002057-BEE-2012 01-01-2014 31/12/2016 TEMPLE,AHMEDABAD 14,NANDANVAN G/AHD/C-2519 SOHAM CONSTRUCTION COMPLEX,OPP.TOWNHALL,ELISH AHMEDABAD G/GS-E-001702-DEE-2012 01-01-201431/12/2016 BRIDGE,AHMEDABAD B-28, ARVIND ESTATE, G/AHD/C/916 YADAV ELECTRICALS BAPUNAGAR CHAR RASTA, AHMEDABAD G/AS/E-10644 01-01-2014 31/12/2016 AHMEDABAD-380025 NEW VAS, PANCHAL VAS, PO. G/AHD/C/785 SHANI ELECTRIC CO AHMEDABAD GAHDSG-06-1-2006 01-01-2014 31/12/2016 SANAND, DIST. AHMEDABAD. 10-A, SHANKARNAGAR SOCIETY, RELIABLE ENGINEERING G/AHD/C/761 NR. GOPI CHOWK, NAVA WADAJ, AHMEDABAD G/AS/E-18750 01-01-2014 31/12/2016 WORK AHMEDABAD-380013. -
RW Ahmedabad March 17
MONTHLY REALTY WATCH Ahmedabad KEY PERFORMANCE INDICATORS March 2017 UP Down No change KEY TRENDS Launches Sales Inventory Prices New launches in Ahmedabad reduced by M-o-M (% change) 58 per cent over the previous month. However, sales registered a growth of 15 58% 15% 1% 0% per cent with nearly 65 per cent contribution from affordable segment Y-o-Y (% change) 58% 36% 10% 2% Localities such as Bopal, Chandkheda, Near Vaishno Devi Circle on SG Highway and Maninagar together accounted for nearly 60 percent of total absorption SECTORWISE M-o-M (% change) witnessed in the city during the month Affordable Mid End Luxury 74% 23% 61% 20% 17% 4% AHMEDABAD Gujarat 2% 3% 1% 0%* 0%* 1%* *Y-o-Y (% change) MONTHLY REALTY WATCH TOP PERFORMING Ahmedabad LOCALITIES March 2017 Gandhinagar Moti Bhoyan Randesan Shantigram Raysan Vadsar Near Vaishno Devi Circle On Ambapur Vaishno SG Highway DeviNear Circle Nirma University on Sanavad SG Highway Chandkheda Rakanpur MOTERA Pardhol Bhadaj CHANDLODIYA Kadadra HANSOL Bilasiya Sardar Patel GHATLODIYA Ring Road Nava Naroda AHMEDABAD Shilaj THAL TEJ MEMNAGAR AIRPORT ASARWA NIKOL Kathwada Pasunj Bopal VASTRAPUR SARASPUR GHUMA ODHAV Bhuvaladi RAMDEV AHMEDABAD NAGAR AMRAIWADI MAKARBA MANINAGAR VASTRAL Maninagar Ahmedabad - Zalod Highway Bakrol ISANPUR Telav Bujrang GIHODASAR VATVA Badrabad Kamod Visalpur Badodara Hirapur Changodar Chosar Map not to Scale 5 4 3 2 1 TOP FIVE LOCALITIES Thaltej Maninagar Near Vaishno Chandkheda Bopal 5,050- 5,450 4,200-5,550 Devi Circle On 3,200-3,400 3,600-3,800 SG Highway 4,100-4,450 (% Contribution to total sales in range during Feb '17) 0-5% 6-10% 11-15% 16-20% Price Range (Rs.