FDA Covid-19 Response at a Glance Summary As of July 19, 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Updated May 26, 2021 Cross-Border Industry Partnerships on COVID-19 Vaccines and Therapeutics Vaccines • Curevac O Celonic Wi
Updated May 26, 2021 Cross-Border Industry Partnerships on COVID-19 Vaccines and Therapeutics Vaccines • CureVac o Celonic will manufacture 100 million doses of CureVac’s vaccine at its plant in Heidelberg, Germany, providing bulk substance for 50 million doses by the end of 2021. (press release) o Novartis will manufacture CureVac’s vaccine. (press release) o GlaxoSmithKline plc and CureVac N.V. announced a new €150m collaboration, building on their existing relationship, to jointly develop next generation mRNA vaccines for COVID-19 with the potential for a multi-valent approach to address multiple emerging variants in one vaccine. (press release) o Rentschler Biopharma SE will manufacture CureVac’s vaccine. (press release) o Bayer will support the further development, supply and key territory operations of CureVac’s vaccine candidate. (press release) o Fareva will dedicate a manufacturing plant in France to the fill and finish of CureVac’s vaccine. (press release) o Wacker Chemie AG will manufacture CureVac’s vaccine candidate at its Amsterdam site. (press release) o CureVac will collaborate with Tesla Grohmann Automation to develop an RNA printer that works like a mini-factory and can produce such drugs automatically. (press release) • Moderna o Samsung Biologics will provide large scale, commercial fill-finish manufacturing for Moderna’s vaccine in South Korea. (press release) o Baxter International will provide fill/finish services and supply packaging for Moderna. (press release) o Sanofi will manufacture 200 million doses of Moderna’s COVID-19 vaccine starting in September 2021. (press release) o Rovi will produce bulk substance for Moderna’s COVID-19 vaccine, expanding an agreement between the companies. -

Miscellaneous Order (07/19/2021)
(ORDER LIST: 594 U.S.) MONDAY, JULY 19, 2021 ORDER IT IS ORDERED that the Court’s orders of March 19, 2020 and April 15, 2020 relating to COVID-19 are rescinded, subject to the clarifications set forth below. IT IS FURTHER ORDERED that, in any case in which the relevant lower court judgment, order denying discretionary review, or order denying a timely petition for rehearing was issued prior to July 19, 2021, the deadline to file a petition for a writ of certiorari remains extended to 150 days from the date of that judgment or order. In any case in which the relevant lower court judgment, order denying discretionary review, or order denying a timely petition for rehearing was issued on or after July 19, 2021, the deadline to file a petition for a writ of certiorari is as provided by Rule 13. IT IS FURTHER ORDERED that the requirement of Rule 33.1 that 40 copies of documents be submitted in booklet format will go back into effect as to covered documents filed on or after September 1, 2021. For submissions pursuant to Rule 33.2, the requirement of Rule 39 that an original and 10 copies be submitted, where applicable, will also go back into effect as to covered documents filed on or after September 1, 2021. The authorization to file a single copy of certain documents on 8½ x 11 inch paper, as set forth in the Court’s April 15, 2020 order, will remain in effect only as to documents filed before September 1, 2021. -

City Council Meeting Agenda July 19, 2021
City Council Meeting Agenda July 19, 2021 The City is working diligently to address the threat posed by COVID-19 (Novel Coronavirus). The City is following the guidance from state and county public health officials to take all efforts to prevent the spread of this virus. In the interest of the safety and welfare of the public, the community, and our employees and to limit the spread of the virus, this City Council meeting will not be open to in-person attendance. Council will be attending this meeting remotely via video conference. All City Council workshops and meetings are broadcast (live closed captioning available) on www.cvtv.org, CVTV cable channels 23 / HD 323, and on the City's Facebook page, www.facebook.com/VancouverUS. In addition, members of the public who wish to listen to the meeting over conference call may do so by calling: 1-866-899-4679 and entering access code 763-551-029 when prompted (credentials unique to this meeting). Council will be accepting remote testimony under Community Communication for the Consent Agenda (comments regarding Item 1-3 on the agenda below only) and under Public Hearings (Items 4-7). Testimony will be accepted in the following manner: Written comments submitted in advance Comments may be sent to [email protected] by 12:00 p.m. on July 19. Comments will be compiled and sent to the City Council and entered into the record. Remote testimony during the meeting Advance registration is required to testify. Anyone wishing to testify on items under the Consent Agenda or Public Hearings below must register by 12:00 p.m. -

Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No
Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No. 33 Class & Market Administrator Payment Dates for Producer Milk Component Final Pool Producer Advance Prices Payment Dates Final Payment Due Partial Payment Due Pool Month Prices Release Date Payrolls Due & Pricing Factors PSF, Admin., MS Cooperative Nonmember Cooperative Nonmember January February 3 * February 13 February 22 December 23, 2020 February 16 ** February 16 February 17 Janaury 25 January 26 February March 3 * March 13 March 22 January 21 * March 15 March 16 March 17 February 25 February 26 March March 31 * April 13 April 22 February 18 * April 15 April 16 April 19 ** March 25 March 26 April May 5 May 13 May 22 March 17 * May 17 ** May 17 ** May 17 April 26 ** April 26 May June 3 * June 13 June 22 April 21 * June 15 June 16 June 17 May 25 May 26 June June 30 * July 13 July 22 May 19 * July 15 July 16 July 19 ** June 25 June 28 ** July August 4 * August 13 August 22 June 23 August 16 ** August 16 August 17 July 26 ** July 26 August September 1 * September 13 September 22 July 21 * September 15 September 16 September 17 August 25 August 26 September September 29 * October 13 October 22 August 18 * October 15 October 18 ** October 18 ** September 27 ** September 27 ** October November 3 * November 13 November 22 September 22 * November 15 November 16 November 17 October 25 October 26 November December 1 * December 13 December 22 October 20 * December 15 December 16 December 17 November 26 ** November 26 December January 5, 2022 January 13, 2022 January 22, 2022 November 17 * January 18, 2022 ** January 18, 2022 ** January 18, 2022 ** December 27 ** December 27 ** * If the release date does not fall on the 5th (Class & Component Prices) or 23rd (Advance Prices & Pricing Factors), the most current release preceding will be used in the price calculation. -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Version 12.0, 01 Jun 2018 ANNEX I SUMMARY of PRODUCT
BioThrax® (Anthrax Vaccine Adsorbed) 1.3.1 Summary of Product Characteristics, Labelling and Package Leaflet Version 12.0, 01 Jun 2018 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS, LABELLING AND PACKAGE LEAFLET Emergent BioSolutions, Inc. Confidential and Proprietary Page 1 of 25 BioThrax® (Anthrax Vaccine Adsorbed) 1.3.1 Summary of Product Characteristics, Labelling and Package Leaflet SUMMARY OF PRODUCT CHARACTERISTICS Emergent BioSolutions, Inc. Confidential and Proprietary Page 2 of 25 BioThrax® (Anthrax Vaccine Adsorbed) 1.3.1 Summary of Product Characteristics, Labelling and Package Leaflet This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See Section 4.8 for how to report adverse reactions 1 NAME OF THE MEDICINAL PRODUCT BioThrax1 suspension for injection. Anthrax Vaccine Adsorbed (purified cell-free filtrate) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One dose (0.5 mL) contains: Anthrax antigen filtrate: 50 micrograms (50 mcg) a, b For a full list of excipients, see Section 6.1. a Produced from cell-free filtrates of an avirulent strain of Bacillus anthracis b Adsorbed on aluminium hydroxide, hydrated (0.6 mg Al3+) 3 PHARMACEUTICAL FORM Suspension for injection. Sterile, milky-white liquid suspension, when mixed. 4 CLINICAL PARTICULARS 4.1 Therapeutic indications BioThrax is indicated for the prevention of disease caused by Bacillus anthracis, in adults at risk of exposure. BioThrax should be used in accordance with official recommendations, where available. 4.2 Posology and method of administration Posology: Primary Immunisation: 3-doses each of 0.5 mL, given at 0, 1 and 6 months. -

Report from Accountable.US
NEW ANALYSIS REVEALS $250M IN INSIDER PROFITEERING AT GOVERNMENT-BACKED DRUG COMPANIES, INCLUDING $105M IN NEW TRADES IN LESS THAN 3 MONTHS’ TIME In Six Months Since Launch Of Operation Warp Speed, Execs At Five Drug Companies Made Net Profit Of More Than $250 Million Dumping Their Companies’ Stocks. An earlier report from Accountable.US compiled executive trading data from the launch of Operation Warp Speed on May 15 through August 31, 2020 and found they made stock transactions valued at a net profit of more than $145 million. New analysis of insider transactions at those companies over the six months between May 15 and November 15, 2020, show more than $250 million in net profit. Company Net Profit Emergent BioSolutions $23,906,515.14 Johnson & Johnson $4,276,630.00 Moderna $165,889,439.69 Novavax $46,372,082.90 Pfizer $10,490,165.85 Total $250,934,833.57 From September To Mid-November, Execs At Four Drug Companies Involved In Operation Warp Speed Have Made Net Profit Of More Than $105M Dumping Their Companies’ Stock. According to SEC filings, from the beginning of September through November 15, 2020, executives and directors at four of the companies receiving COVID vaccine funding through Operation Warp Speed have made stock transactions valued at a net profit of more than $105 million. NOTE: Johnson & Johnson did not report any new individual insider transactions during this period. Company Net Profit Emergent BioSolutions $18,860,527.37 Moderna $50,406,146.77 Novavax $29,037,668.05 Pfizer $7,388,569.80 Total $105,692,911.99 • The net profit of these transactions from May 15-November 15, 2020 was $250.9 million; the total profit of these trades was $292.1 million. -

Online Permit Upgrades Coming July 19 and New Webinars in July ______
CITY OF SAN DIEGO - 1222 FIRST AVE., M.S. 501, SAN DIEGO, CA 92101 PUBLIC NOTICE _____________________________________________________________________________________________ Date: July 2, 2021 Subject: Online Permit Upgrades Coming July 19 and New Webinars in July _____________________________________________________________________________________________ Starting Monday, July 19, the Development Services Department (DSD) will be updating the online permit process for all Building and Engineering permit types. This central system update will transform the "hybrid submittal process" launched during the COVID- 19 pandemic to an entirely cloud-based permitting system that will provide convenient 24/7 access to customers. This transition will reduce operational delays processing permits. The new online permitting system offers many benefits that will enhance customer transactions, making it more intuitive, easier and faster for customers to apply for a new permit, track projects during the review, schedule inspections and make online payments. Benefits include eliminating the two-step application process, separate requests for PTS numbers prior to document upload, and the general application form. The new system will make it easier to add and upload new documents, conduct cloud-based plan reviews and incorporating plan review comments for customers to view in real-time. Important! System Upgrade Timeline To accommodate the system updates, intake for new building and engineering applications will be suspended from July 13 to July 19. Customers are advised to plan accordingly using the timeline below. • July 12 - Last Day to Submit New Applications Using Current Process To accommodate the critical update, Monday, July 12, at 5 p.m. will be the last day Online Permitting Upgrades Coming July 19 Page 2 to submit new Building and Engineering applications using the current hybrid submittal process. -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
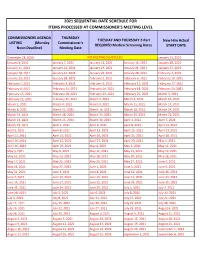
2021 Sequential Date List
2021 SEQUENTIAL DATE SCHEDULE FOR ITEMS PROCESSED AT COMMISSIONER'S MEETING LEVEL COMMISSIONERS AGENDA THURSDAY TUESDAY AND THURSDAY 2-Part New Hire Actual LISTING (Monday Commissioner's REQUIRED Medical Screening Dates START DATE Noon Deadline) Meeting Date December 28, 2020 NO MEETING SCHEDULED January 13, 2021 January 4, 2021 January 7, 2021 January 12, 2021 January 14, 2021 January 20, 2021 January 11, 2021 January 14, 2021 January 19, 2021 January 21, 2021 January 27, 2021 January 18, 2021 January 21, 2021 January 26, 2021 January 28, 2021 February 3, 2021 January 25, 2021 January 28, 2021 February 2, 2021 February 4, 2021 February 10, 2021 February 1, 2021 February 4, 2021 February 9, 2021 February 11, 2021 February 17, 2021 February 8, 2021 February 11, 2021 February 16, 2021 February 18, 2021 February 24, 2021 February 15, 2021 February 18, 2021 February 23, 2021 February 25, 2021 March 3, 2021 February 22, 2021 February 25, 2021 March 2, 2021 March 4, 2021 March 10, 2021 March 1, 2021 March 4, 2021 March 9, 2021 March 11, 2021 March 17, 2021 March 8, 2021 March 11, 2021 March 16, 2021 March 18, 2021 March 24, 2021 March 15, 2021 March 18, 2021 March 23, 2021 March 25, 2021 March 31, 2021 March 22, 2021 March 25, 2021 March 30, 2021 April 1, 2021 April 7, 2021 March 29, 2021 April 1, 2021 April 6, 2021 April 8, 2021 April 14, 2021 April 5, 2021 April 8, 2021 April 13, 2021 April 15, 2021 April 21, 2021 April 12, 2021 April 15, 2021 April 20, 2021 April 22, 2021 April 28, 2021 April 19, 2021 April 22, 2021 April 27, 2021 April -

2021 Subdivision Calendar
Planning Department Development Review – Major Subdivision Applications First Plat Review 2021 Deadlines Submittal to Staff Sufficiency TRC Meeting Date Deadline for Staff Planning Board BOC Meeting Planning Department Review Deadline Postponement Meeting Date* Date** December 18, 2020 December 31 January 13 January 22 February 2 March 15 January 15 January 29 February 10 February 19 March 2 April 19 February 19 March 5 March 17 March 26 April 6 May 17 March 19 April 2 April 14 April 23 May 4 June 21 April 16 April 30 May 12 May 21 June 1 July 19 May 21 June 4 June 16 June 25 July 6 August 16 June 18 July 2 July 14 July 23 August 3 September 20 July 23 August 6 August 18 August 27 September 7 October 18 August 20 September 3 September 15 September 24 October 5 November 15 September 17 October 1 October 13 October 22 November 2 December 20 October 22 November 5 November 17 November 26 December 7 ▪ January 18, 2022 November 19 December 3 December 15 ▪ December 22 ▪ January 4, ▪ February 21, 2022 2022 ▪ ▪ ▪ December 17 December 31, January 12, ▪ January 21, ▪ February 1, March 21, 2021 2022 2022 2022 2022 * Planning Board Has Two (2) Meetings to Make Recommendation ** Board of Commissioners Has Up to Four (4) Meetings to Make Decision ▪ 2021 Dates are Tentative – Meeting Dates Will Be Set in December 2021 With Adoption of 2022 Meeting Calendars Construction/Final Plat Review – Administrative* 2021 Deadlines Submittal to Staff Sufficiency TRC Meeting Date Planning Review Deadline Department For TRC December 23, 2020 January 6 January 13 January