NZPHR Vol 8 No 10 Oct 2001
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Police Deny Claim Gangs Here in Large Numbers
Issue 186 Helensville News March 2016 5000 copies delivered monthly to Helensville, Parakai, Kaukapakapa, Waitoki, Wainui, Woodhill, South Head and Shelly Beach Police deny claim gangs here in large numbers Police have refuted a claim by New Zealand First deputy leader and reintegration to address gangs and transnational crime groups. and police spokesperson Ron Mark that more than 70 high ranking “[It] is not just about enforcement,” says Inspector Fergus. “It's also gang members have moved into the Helensville and Kumeu areas. about strengthening families and inspiring the children at the bottom of Mr Mark made the claim during parliamentary question time on the family tree, creating different and positive pathways, and reducing February 16 and in a subsequent press release, saying the gang the harms gangs disproportionately represent. This is long-haul, inter- members have moved here “as a direct result of police under- generational work.” resourcing.” He says that locally, police work on a number of levels to reduce But Inspector Mark Fergus, Rodney Area Commander for the the harm caused by gang members. police, says: “Our intelligence does not support [Mr Mark’s] statement “This includes enforcement of road rules, monitoring motorcycle that ‘over 70 high ranking gang members’ reside in Helensville and gang runs, and gathering intelligence to pass to our organised crime Kumeu. units.” “However, we are aware of the presence of patched outlaw Inspector Fergus says members of the public should speak to their motorcycle gang members residing in these communities, as they do local Community Constable or call Crimestoppers anonymously on in many other communities across the country, both urban and rural.” 0800 555 111 about suspicious activities or movements of patched Mr Mark questioned the Minister of Police, Judith Collins in gang members. -

Q Fever in Small Ruminants and Its Public Health Importance
Journal of Dairy & Veterinary Sciences ISSN: 2573-2196 Review Article Dairy and Vet Sci J Volume 9 Issue 1 - January 2019 Copyright © All rights are reserved by Tolera Tagesu Tucho DOI: 10.19080/JDVS.2019.09.555752 Q Fever in Small Ruminants and its Public Health Importance Tolera Tagesu* School of Veterinary Medicine, Jimma University, Ethiopia Submission: December 01, 2018; Published: January 11, 2019 *Corresponding author: Tolera Tagesu Tucho, School of Veterinary Medicine, Jimma University, Jimma Oromia, Ethiopia Abstract Query fever is caused by Coxiella burnetii, it’s a worldwide zoonotic infectious disease where domestic small ruminants are the main reservoirs for human infections. Coxiella burnetii, is a Gram-negative obligate intracellular bacterium, adapted to thrive within the phagolysosome of the phagocyte. Humans become infected primarily by inhaling aerosols that are contaminated with C. burnetii. Ingestion (particularly drinking raw milk) and person-to-person transmission are minor routes. Animals shed the bacterium in urine and feces, and in very high concentrations in birth by-products. The bacterium persists in the environment in a resistant spore-like form which may become airborne and transported long distances by the wind. It is considered primarily as occupational disease of workers in close contact with farm animals or processing their be commenced immediately whenever Q fever is suspected. To prevent both the introduction and spread of Q fever infection, preventive measures shouldproducts, be however,implemented it may including occur also immunization in persons without with currently direct contact. available Doxycycline vaccines drugof domestic is the first small line ruminant of treatment animals for Q and fever. -

Zoonotic Diseases of Public Health Importance
ZOONOTIC DISEASES OF PUBLIC HEALTH IMPORTANCE ZOONOSIS DIVISION NATIONAL INSTITUTE OF COMMUNICABLE DISEASES (DIRECTORATE GENERAL OF HEALTH SERVICES) 22 – SHAM NATH MARG, DELHI – 110 054 2005 List of contributors: Dr. Shiv Lal, Addl. DG & Director Dr. Veena Mittal, Joint Director & HOD, Zoonosis Division Dr. Dipesh Bhattacharya, Joint Director, Zoonosis Division Dr. U.V.S. Rana, Joint Director, Zoonosis Division Dr. Mala Chhabra, Deputy Director, Zoonosis Division FOREWORD Several zoonotic diseases are major public health problems not only in India but also in different parts of the world. Some of them have been plaguing mankind from time immemorial and some have emerged as major problems in recent times. Diseases like plague, Japanese encephalitis, leishmaniasis, rabies, leptospirosis and dengue fever etc. have been major public health concerns in India and are considered important because of large human morbidity and mortality from these diseases. During 1994 India had an outbreak of plague in man in Surat (Gujarat) and Beed (Maharashtra) after a lapse of around 3 decades. Again after 8 years in 2002, an outbreak of pneumonic plague occurred in Himachal Pradesh followed by outbreak of bubonic plague in 2004 in Uttaranchal. Japanese encephalitis has emerged as a major problem in several states and every year several outbreaks of Japanese encephalitis are reported from different parts of the country. Resurgence of Kala-azar in mid seventies in Bihar, West Bengal and Jharkhand still continues to be a major public health concern. Efforts are being made to initiate kala-azar elimination programme by the year 2010. Rabies continues to be an important killer in the country. -

12 December 19
Issue 228 Helensville News December 2019 5000 copies delivered monthly to Helensville, Parakai, Kaukapakapa, Waitoki, Wainui, Woodhill, South Head and Shelly Beach A quick ride - right around the country Deciding to ride a horse around the 12 months to complete the ride. entire country when you have no In Helensville, riding up Karaka experience as a rider might seem a Street, she was stopped by a woman little foolhardy. who ran out of her house to give her a But for 31-year-old Larissa snack bag for the journey. Mueller, who rode through the South She receives regular offers of Kaipara area recently on her way to meals and places to overnight, doing just that, it’s a way to raise sleeping in spare rooms, sleepouts, money for disadvantaged youth. caravans, barns or whatever is on Larissa and her friend Kendall offer. She carries a small tent for the Waugh decided to go shares in a horse nights she can’t find a roof to sleep back in 2015, even though Larissa had under - about half the time. only been on a horse a handful of times “With my background in outdoor as a youngster. During the course of instructing I can sleep just about that same afternoon, the decision grew anywhere,” Larissa says. to encompass a ride around the entire “My priority is finding a paddock coastline of New Zealand. for Sprite. I have never yet had to “The ride came first. We decided to tether her, and hope never to. The do the ride before we heard about the people of New Zealand are so Leg-Up Trust,” says Larissa. -

September 2019
Issue 225 Helensville News September 2019 5000 copies delivered monthly to Helensville, Parakai, Kaukapakapa, Waitoki, Wainui, Woodhill, South Head and Shelly Beach Defibrilators availablelocally 24/7 Helensville St John is partnering with members would like local service organisations to install publicly to talk to any local available Automated External Defibrillators organisations or (AEDs) in easily accessible locations around businesses that our area. would be willing to The Helensville St John Area Committee have an AED has already installed the first one beside the attached to the main doors of the Helensville RSA, in central outside wall of their Commercial Road. premises. This is the first stage of a roll-out of more Now there are a units providing coverage from Kumeu to couple in central Glorit and out to South Head. Helensville, they are The Lions Club of Helensville have looking further afield. purchased an AED from St John, for Ideally sites should installation at the Rautawhiri Park tennis club be on a south facing rooms, and South Kaipara Rotary are also wall and have a looking to assist with theAED roll-out. canopy or similar The AEDs are available 24 hours a day, cover. seven days a week. Full instructions for use The units and are on the outside of the cover. To use one, a their covers are very person must first phone 111 so the St John robust, but direct communications staff can help with sunlight can speed unlocking the alarmed cover. up the need for ! Helensville St John Area Committee treasurer John Issott (left) with RSA Once the cabinet is unlocked, the AED replacement. -
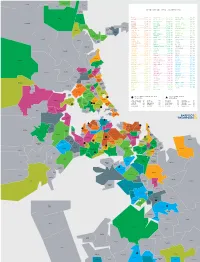
TOP MEDIAN SALE PRICE (OCT19—SEP20) Hatfields Beach
Warkworth Makarau Waiwera Puhoi TOP MEDIAN SALE PRICE (OCT19—SEP20) Hatfields Beach Wainui EPSOM .............. $1,791,000 HILLSBOROUGH ....... $1,100,000 WATTLE DOWNS ......... $856,750 Orewa PONSONBY ........... $1,775,000 ONE TREE HILL ...... $1,100,000 WARKWORTH ............ $852,500 REMUERA ............ $1,730,000 BLOCKHOUSE BAY ..... $1,097,250 BAYVIEW .............. $850,000 Kaukapakapa GLENDOWIE .......... $1,700,000 GLEN INNES ......... $1,082,500 TE ATATŪ SOUTH ....... $850,000 WESTMERE ........... $1,700,000 EAST TĀMAKI ........ $1,080,000 UNSWORTH HEIGHTS ..... $850,000 Red Beach Army Bay PINEHILL ........... $1,694,000 LYNFIELD ........... $1,050,000 TITIRANGI ............ $843,000 KOHIMARAMA ......... $1,645,500 OREWA .............. $1,050,000 MOUNT WELLINGTON ..... $830,000 Tindalls Silverdale Beach SAINT HELIERS ...... $1,640,000 BIRKENHEAD ......... $1,045,500 HENDERSON ............ $828,000 Gulf Harbour DEVONPORT .......... $1,575,000 WAINUI ............. $1,030,000 BIRKDALE ............. $823,694 Matakatia GREY LYNN .......... $1,492,000 MOUNT ROSKILL ...... $1,015,000 STANMORE BAY ......... $817,500 Stanmore Bay MISSION BAY ........ $1,455,000 PAKURANGA .......... $1,010,000 PAPATOETOE ........... $815,000 Manly SCHNAPPER ROCK ..... $1,453,100 TORBAY ............. $1,001,000 MASSEY ............... $795,000 Waitoki Wade HAURAKI ............ $1,450,000 BOTANY DOWNS ....... $1,000,000 CONIFER GROVE ........ $783,500 Stillwater Heads Arkles MAIRANGI BAY ....... $1,450,000 KARAKA ............. $1,000,000 ALBANY ............... $782,000 Bay POINT CHEVALIER .... $1,450,000 OTEHA .............. $1,000,000 GLENDENE ............. $780,000 GREENLANE .......... $1,429,000 ONEHUNGA ............. $999,000 NEW LYNN ............. $780,000 Okura Bush GREENHITHE ......... $1,425,000 PAKURANGA HEIGHTS .... $985,350 TAKANINI ............. $780,000 SANDRINGHAM ........ $1,385,000 HELENSVILLE .......... $985,000 GULF HARBOUR ......... $778,000 TAKAPUNA ........... $1,356,000 SUNNYNOOK ............ $978,000 MĀNGERE ............. -

Biannual Communicable Disease Surveillance Report #2
BIANNUAL COMMUNICABLE DISEASE SURVEILLANCE REPORT #2 An overview of the public health nurse surveillance of THE TOWN communicable diseases in Grafton, MA, Jan 1 – Dec 31, 2020. 2020OF GRAFTON Grafton Biannual Communicable Disease Surveillance Report #2, 2020 Defining Case Classifications for Communicable Diseases In the U.S., the States mandate the reporting of certain diseases by law or by regulation. The diseases that are reportable to state and local health departments differ from state to state; however, certain diseases are considered nationally notifiable diseases. The list of nationally notifiable diseases is updated annually. The Centers for Disease Control and Prevention (CDC), in collaboration with the Council of State and Territorial Epidemiologists (CSTE), publishes case definitions for public health surveillance for the nationally notifiable diseases. These case definitions provide uniform criteria for reporting cases and are case specific. The case status for most diseases is determined as follows: • A confirmed case is one in which the clinical case description is met and the laboratory confirmation requirement is met. A case may also be considered confirmed if it is linked to a laboratory-confirmed case. Certain diseases may not include laboratory findings as testing is not available. • A probable case is one in which the clinical case description is met and there is supportive or presumptive laboratory results consistent with the diagnosis but, it does not meet the laboratory confirmed criteria. • A suspect case is one in which the clinical case description is met. • A revoked case is one in which neither the suspect, probable, nor confirmed case definition is met. A significant amount of information gathering must be collected for many diseases before a case classification is final. -

61% of All Human Pathogens Are Zoonotic (Passed from Animals to Humans), and Many Are Transmitted Through Inhaling Dust Particles Or Contact with Animal Wastes
Zoonotic Diseases Fast Facts: 61% of all human pathogens are zoonotic (passed from animals to humans), and many are transmitted through inhaling dust particles or contact with animal wastes. Some of the diseases we can get from our pets may be fatal if they go undetected or undiagnosed. All are serious threats to human health, but can usually be avoided by observing a few precautions, the most effective of which is washing your hands after touching animals or their wastes. Regular visits to the veterinarian for prevention, diagnosis, and treatment of zoonotic diseases will help limit disease in your pet. Source: http://www.cdc.gov/healthypets/ Some common zoonotic diseases humans can get through their pets: Zoonotic Disease & its Effect on How Contact is Made Humans Bartonellosis (cat scratch disease) – an Bartonella bacteria are transferred to humans through infection from the bacteria Bartonella a bite or scratch. Do not play with stray cats, and henselae that causes fever and swollen keep your cat free of fleas. Always wash hands after lymph nodes. handling your cat. Capnocytophaga infection – an Capnocytophaga canimorsus is the main human infection caused by bacteria that can pathogen associated with being licked or bitten by an develop into septicemia, meningitis, infected dog and may present a problem for those and endocarditis. who are immunosuppressed. Cellulitis – a disease occurring when Bacterial organisms from the Pasteurella species live bacteria such as Pasteurella multocida in the mouths of most cats, as well as a significant cause a potentially serious infection of number of dogs and other animals. These bacteria the skin. -

Enteric Infections Due to Campylobacter, Yersinia, Salmonella, and Shigella*
Bulletin of the World Health Organization, 58 (4): 519-537 (1980) Enteric infections due to Campylobacter, Yersinia, Salmonella, and Shigella* WHO SCIENTIFIC WORKING GROUP1 This report reviews the available information on the clinical features, pathogenesis, bacteriology, and epidemiology ofCampylobacter jejuni and Yersinia enterocolitica, both of which have recently been recognized as important causes of enteric infection. In the fields of salmonellosis and shigellosis, important new epidemiological and relatedfindings that have implications for the control of these infections are described. Priority research activities in each ofthese areas are outlined. Of the organisms discussed in this article, Campylobacter jejuni and Yersinia entero- colitica have only recently been recognized as important causes of enteric infection, and accordingly the available knowledge on these pathogens is reviewed in full. In the better- known fields of salmonellosis (including typhoid fever) and shigellosis, the review is limited to new and important information that has implications for their control.! REVIEW OF RECENT KNOWLEDGE Campylobacterjejuni In the last few years, C.jejuni (previously called 'related vibrios') has emerged as an important cause of acute diarrhoeal disease. Although this organism was suspected of being a cause ofacute enteritis in man as early as 1954, it was not until 1972, in Belgium, that it was first shown to be a relatively common cause of diarrhoea. Since then, workers in Australia, Canada, Netherlands, Sweden, United Kingdom, and the United States of America have reported its isolation from 5-14% of diarrhoea cases and less than 1 % of asymptomatic persons. Most of the information given below is based on conclusions drawn from these studies in developed countries. -

Agenda of Rodney Local Board
I hereby give notice that an ordinary meeting of the Rodney Local Board will be held on: Date: Wednesday 15 July 2020 Time: 3.00pm Meeting Room: Te Whare Oranga ō Parakai Venue: 5 Rere Place Parakai Rodney Local Board OPEN AGENDA MEMBERSHIP Chairperson Phelan Pirrie Deputy Chairperson Beth Houlbrooke Members Brent Bailey Steve Garner Danielle Hancock Tim Holdgate Louise Johnston Vicki Kenny Colin Smith (Quorum 5 members) Robyn Joynes Democracy Advisor - Rodney 10 July 2020 Contact Telephone: +64 212447174 Email: [email protected] Website: www.aucklandcouncil.govt.nz Note: The reports contained within this agenda are for consideration and should not be construed as Council policy unless and until adopted. Should Members require further information relating to any reports, please contact the relevant manager, Chairperson or Deputy Chairperson. Board Member Organisation Position Brent Bailey Royal NZ Yacht Squadron Member Steven Garner Warkworth Tennis and Squash Club President Sandspit Yacht Club Member Warkworth Gamefish Club Member Louise Johnston Blackbridge Environmental Protection Treasurer Society Vicki Kenny International Working Holidays Ltd Director/Owner/CEO Nannies Abroad Ltd Director/Owner/CEO Waitemata Riding Club Member Treasurer National Party Helensville Electorate Danielle Hancock Kaukapakapa Residents and Ratepayers Member Association Pest Free Kaukapakapa Pest Free Coordinator New Zealand Biosecurity Services Limited Operations Manager Tim Holdgate Landowners Contractors Protection Vice Chairman Association -

Salmonella Is One of the Most Common Foodborne Infections
Salmonellosis is one of the most common foodborne infections in the United States, resulting in an estimated 1.2 million human cases and $365 million in direct medical costs annually (2011 estimates). Signs and Symptoms When Salmonella bacteria are ingested, they pass through a person’s stomach and colonize the small and large intestine. There, the bacteria invade the intestinal mucosa and proliferate. The bacteria can invade the lymphoid tissues of the gastrointestinal tract and spread to the bloodstream. Dissemination to the bloodstream depends on host factors and virulence of the Salmonella strain and occurs in less than 5% of infections. If the infection spreads to the bloodstream, any organ can become infected (e.g., liver, gallbladder, bones, or meninges). The incubation period for salmonellosis is approximately 12–72 hours, but it can be longer. Salmonella gastroenteritis is characterized by the sudden onset of • diarrhea (sometime blood-tinged), • abdominal cramps • fever, and • occasionally nausea and vomiting. Illness usually lasts 4–7 days. If the infection spreads to the bloodstream and distant organs, the illness increases in duration and severity and will usually include signs and symptoms related to the organ affected. A small proportion of persons infected with Salmonella develop reactive arthritis as a long-term sequela of the infection. Diagnosis Multiple diseases can cause fever, diarrhea, and abdominal cramps. Therefore, salmonellosis cannot be diagnosed on the basis of symptoms alone. To diagnose salmonellosis, the bacterium is usually isolated in the laboratory from the patient's stool. The genus Salmonella is identified by using a series of biochemical tests. Subtyping (e.g., serotyping, pulsed field gel electrophoresis, and other tests) and antimicrobial susceptibility testing of Salmonella isolates are important adjuncts to the diagnostic testing of patients. -

February 2011
Issue 130 Helensville News February 2011 4600 copies delivered monthly to Helensville, Parakai, Kaukapakapa, Waitoki, Wainui, Woodhill, South Head and Shelly Beach Guide camp boon for local business ! Part of the Girl Guides ‘Kaipara Kapers’ camp at the Helensville Showgrounds The Helensville Showgrounds was Auckland (which includes the Helensville themselves to the ropes courses at Tree turned into a tent village last month - and the area), Manukau and Hauraki - and spans Adventures in Woodhill, kayaking at Shelly local economy given a boost - by the first the upper half of the North Island, from Beach, and going 'geocaching', which is like ever Girl Guides Upper North Zone camp. Kaitaia to the Hauraki Plains. a modern-day treasure hunt using GPS 267 Girl Guides aged from 10 years, All the girls came from within that area, technology. plus 36 Girl Guide Rangers (aged 14-18) as did all but one of the leaders, a Canadian On the Saturday afternoon, the girls took part in the five-day 'Kaipara Kapers' woman holidaying here who immediately took part in a number of local community event. They were looked after by 75 volunteered to help after hearing about the service projects. volunteer adults - only three of whom, the camp. Those included washing the Kaipara bus driver, advanced paramedic and Helensville Showgrounds was chosen Coastguard boat, gardening and general security guard - were men. for its central location in the tidying up at both the RSA's The event took 18 months to organise Zone, its large size, and the ‘Running the camp was akin Drake Village and Mt Tabor and cost $98,000 to run, with each girl fact it is in a rural area yet Trust, helping with Lions contributing an all-inclusive $290 and the close to amenities and to a military exercise.’ Club fundraising by pulling adult leaders $110 each.