Ariidae Mugilidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Belonidae Bonaparte 1832 Needlefishes
ISSN 1545-150X California Academy of Sciences A N N O T A T E D C H E C K L I S T S O F F I S H E S Number 16 September 2003 Family Belonidae Bonaparte 1832 needlefishes By Bruce B. Collette National Marine Fisheries Service Systematics Laboratory National Museum of Natural History, Washington, DC 20560–0153, U.S.A. email: [email protected] Needlefishes are a relatively small family of beloniform fishes (Rosen and Parenti 1981 [ref. 5538], Collette et al. 1984 [ref. 11422]) that differ from other members of the order in having both the upper and the lower jaws extended into long beaks filled with sharp teeth (except in the neotenic Belonion), the third pair of upper pharyngeal bones separate, scales on the body relatively small, and no finlets following the dorsal and anal fins. The nostrils lie in a pit anterior to the eyes. There are no spines in the fins. The dorsal fin, with 11–43 rays, and anal fin, with 12–39 rays, are posterior in position; the pelvic fins, with 6 soft rays, are located in an abdominal position; and the pectoral fins are short, with 5–15 rays. The lateral line runs down from the pectoral fin origin and then along the ventral margin of the body. The scales are small, cycloid, and easily detached. Precaudal vertebrae number 33–65, caudal vertebrae 19–41, and total verte- brae 52–97. Some freshwater needlefishes reach only 6 or 7 cm (2.5 or 2.75 in) in total length while some marine species may attain 2 m (6.5 ft). -

Fish and Mammals in the Economy of an Ancient Peruvian Kingdom
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 6564–6570, May 1999 Anthropology This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 29, 1997. Fish and mammals in the economy of an ancient Peruvian kingdom JOYCE MARCUS*, JEFFREY D. SOMMER, AND CHRISTOPHER P. GLEW Museum of Anthropology, 1109 Geddes Avenue, University of Michigan, Ann Arbor, MI 48109-1079 Contributed by Joyce Marcus, April 8, 1999 ABSTRACT Fish and mammal bones from the coastal Yunga and Chaupi Yunga site of Cerro Azul, Peru shed light on economic specialization just before the Inca conquest of A.D. 1470. The site devoted In Quechua, the language of the Inca, the Peruvian coast was itself to procuring anchovies and sardines in quantity for divided into yunga and chaupi yunga. The yunga, or coast shipment to agricultural communities. These small fish were proper, is an arid strip along the ocean, rarely extending inland dried, stored, and eventually transported inland via caravans more than 50 km. Beyond that point, it merges with the chaupi of pack llamas. Cerro Azul itself did not raise llamas but yunga, a piedmont zone at the base of the Andes. The chaupi obtained charqui (or dried meat) as well as occasional whole yunga is cut by stream canyons supporting trees, shrubs, and adult animals from the caravans. Guinea pigs were locally grasses that are rare to absent in the yunga. raised. Some 20 species of larger fish were caught by using In ancient times, one could hunt deer and guanaco in these nets; the more prestigious varieties of these show up mainly in piedmont canyons. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

(Galeichthys Peruvianus) Otoliths
THE UNIVERSITY OF ALABAMA University Libraries Oxygen Isotope Record of the 1997–1998 El Niño in Peruvian Sea Catfish (Galeichthys peruvianus) Otoliths C. Fred T. Andrus – University of Georgia Douglas E. Crowe – University of Georgia Christopher S. Romanek – University of Georgia Deposited 01/16/2018 Citation of published version: Andrus, C., Crowe, D., Romanek, C. (2002): Oxygen Isotope Record of the 1997–1998 El Niño in Peruvian Sea Catfish (Galeichthys peruvianus) Otoliths. Paleoceanography, 17(4). DOI: https://doi.org/10.1029/2001PA000652 © 2002. American Geophysical Union. All Rights Reserved. PALEOCEANOGRAPHY, VOL. 17, NO. 4, 1053, doi:10.1029/2001PA000652, 2002 Oxygen isotope record of the 1997–1998 El Nin˜o in Peruvian sea catfish (Galeichthys peruvianus) otoliths C. Fred T. Andrus, Douglas E. Crowe, and Christopher S. Romanek1 Department of Geology, University of Georgia, Athens, Georgia, USA Received 2 May 2001; revised 9 January 2002; accepted 23 January 2002; published 11 October 2002. [1] Sagittal otoliths of the Peruvian sea catfish Galeichthys peruvianus were collected from the north coast of Peru during and after the 1997–1998 El Nin˜o. The otoliths were analyzed via laser microprobe and micromilling techniques for oxygen isotope composition through ontogeny to document their use as an El Nin˜o-Southern Oscillation (ENSO) proxy. Results were compared to theoretical calculations for the d18O of otolith aragonite using measured sea surface temperatures (SST) and d18O values for local seawater assuming equilibrium oxygen isotope fractionation was achieved. All otoliths recorded the 1997–1998 El Nin˜o event as well as seasonal temperature variations. These ENSO events were recorded in otolith aragonite as significant negative excursions in d18O that reflected the increased temperature of local marine waters. -
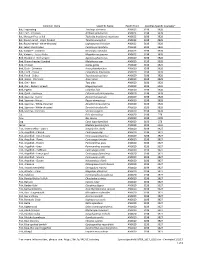
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -
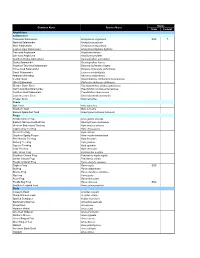
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

A Checklist of Texas Fresh-Water Fishes By
A Checklist of I Texas Fresh-Water Fishes By CLARK HUBBS Department of Zoology The University of Texas DIVISION OF INLAND FISHERIES TEXAS GAME AND FISH COMMISSION Austin, Texas Marion Toole, Director IF Series - No. 3 Revised Dec. 1958 FOREWORD A checklist of Texas fresh-water fishes by CLARK HUBBS This checklist is modified from that of Hubbs (1957a). A number of changes have been made in nomenclature. Notropis roseus and N. deliciosus have been changed to N. texanus and N. strarnineus, re spectively, following Suttkus (1958). Etheostorna whipplei and E. m·tesiae have been changed to E. radiosum following a re-examina tion of available material. Two species have been added, Garnbusia senilis, following Hubbs (1958), and Eucinoslornus argenteus, a marine species collected in a .coastal stream near Brownsville. Two species which had not been described in the previous checklist are given their names, Gambusia geiseri, following Hubbs and Springer (1957), and G. helerochir, following Hubbs (1957b). The primary difference between the checklists is the addition of information on the distribution of fishes within the state to this list. The general concepts follow those given in a previous report (Hubbs, 1957c), but emphasize the ranges of the individual species rather than the distributional patterns. The range designations follow the common names of all species. The numbers refer to the modified game areas shovvn on the map. If the fish inhabits only a part of the area, it is so designated by preceding the number with letters (N. for north, etc.) indicating· the pai·t of the area inhabited by the species. -

Changes in the Fish Community of a Western Caribbean Estuary After the Expansion of an Artificial Channel to the Sea
water Article Changes in the Fish Community of a Western Caribbean Estuary after the Expansion of an Artificial Channel to the Sea Juan J. Schmitter-Soto * and Roberto L. Herrera-Pavón El Colegio de la Frontera Sur, Av. Centenario km 5.5, Chetumal 77014, Quintana Roo, Mexico; [email protected] * Correspondence: [email protected]; Tel.: +52-983-835-0440 (ext. 4302) Received: 30 October 2019; Accepted: 2 December 2019; Published: 6 December 2019 Abstract: Increased connectivity between coastal lagoons and the sea is expected to entail a greater proportion of marine species in the former. Chetumal Bay, estuary of the Hondo river into the Caribbean, had a limited access to the sea until the opening of the Zaragoza Canal. We sought changes in the fish community from 1999–2001 (just after an expansion of the canal) to 2015–2018. The same fishing gear was used, in the same localities, during all seasons. Total fish abundance and mean local richness decreased, although total abundance increased in the polyhaline zone. Diversity was greater in the oligohaline zone in 1999–2001, and in the mesohaline zone in 2015–2018. Three guilds were absent in 2015–2018: Medium-sized herbivores, large piscivores, and medium-sized planktivores. Abundance of small benthivores decreased by decade; medium-sized piscivores and small planktivores became more abundant in 2015–2018 in the polyhaline zone. These changes may be due to the opening of the channel, but illegal fishing outside the bay may explain the decrease in juveniles of large piscivores, and erosion in the innermost part may be destroying important habitats. -

FISHES (C) Val Kells–November, 2019
VAL KELLS Marine Science Illustration 4257 Ballards Mill Road - Free Union - VA - 22940 www.valkellsillustration.com [email protected] STOCK ILLUSTRATION LIST FRESHWATER and SALTWATER FISHES (c) Val Kells–November, 2019 Eastern Atlantic and Gulf of Mexico: brackish and saltwater fishes Subject to change. New illustrations added weekly. Atlantic hagfish, Myxine glutinosa Sea lamprey, Petromyzon marinus Deepwater chimaera, Hydrolagus affinis Atlantic spearnose chimaera, Rhinochimaera atlantica Nurse shark, Ginglymostoma cirratum Whale shark, Rhincodon typus Sand tiger, Carcharias taurus Ragged-tooth shark, Odontaspis ferox Crocodile Shark, Pseudocarcharias kamoharai Thresher shark, Alopias vulpinus Bigeye thresher, Alopias superciliosus Basking shark, Cetorhinus maximus White shark, Carcharodon carcharias Shortfin mako, Isurus oxyrinchus Longfin mako, Isurus paucus Porbeagle, Lamna nasus Freckled Shark, Scyliorhinus haeckelii Marbled catshark, Galeus arae Chain dogfish, Scyliorhinus retifer Smooth dogfish, Mustelus canis Smalleye Smoothhound, Mustelus higmani Dwarf Smoothhound, Mustelus minicanis Florida smoothhound, Mustelus norrisi Gulf Smoothhound, Mustelus sinusmexicanus Blacknose shark, Carcharhinus acronotus Bignose shark, Carcharhinus altimus Narrowtooth Shark, Carcharhinus brachyurus Spinner shark, Carcharhinus brevipinna Silky shark, Carcharhinus faiformis Finetooth shark, Carcharhinus isodon Galapagos Shark, Carcharhinus galapagensis Bull shark, Carcharinus leucus Blacktip shark, Carcharhinus limbatus Oceanic whitetip shark, -

Molecular Clocks Provide New Insights Into the Evolutionary History of Galeichthyine Sea Catfishes
ORIGINAL ARTICLE doi:10.1111/j.1558-5646.2009.00640.x MOLECULAR CLOCKS PROVIDE NEW INSIGHTS INTO THE EVOLUTIONARY HISTORY OF GALEICHTHYINE SEA CATFISHES Ricardo Betancur-R.1,2 and Jonathan W. Armbruster1 1Department of Biological Sciences, Auburn University, 331 Funchess Hall, Auburn, Alabama 36849 2E-mail: [email protected] Received August 28, 2008 Accepted January 7, 2009 Intercontinental distributions in the southern hemisphere can either be the result of Gondwanan vicariance or more recent transoceanic dispersal. Transoceanic dispersal has come into vogue for explaining many intercontinental distributions; however, it has been used mainly for organisms that can float or raft between the continents. Despite their name, the Sea Catfishes (Ariidae) have limited dispersal ability, and there are no examples of nearshore ariid genera with a transoceanic distribution except for Galeichthys where three species occur in southern Africa and one in the Peruvian coast. A previous study suggested that the group originated in Gondwana, and that the species arrived at their current range after the breakup of the supercontinent in the Early Cretaceous. To test this hypothesis, we infer molecular phylogenies (mitochondrial cytochrome b, ATP synthase 8/6, 12S, and 16S; nuclear rag2; total ∼4 kb) and estimate intercontinental divergence via molecular clocks (penalized-likelihood, Bayesian relaxed clock, and universal clock rates in fishes). Age ranges for cladogenesis of African and South American lineages are 15.4–2.5 my, far more recent than would be suggested by Gondwanan vicariance; thus, the distribution of galeichthyines must be explained by dispersal or more recent vicariant events. The nested position of the Peruvian species (Galeichthys peruvianus) within the African taxa is robust, suggesting that the direction of the dispersal was from Africa to South America. -

Multi-Locus Fossil-Calibrated Phylogeny of Atheriniformes (Teleostei, Ovalentaria)
Molecular Phylogenetics and Evolution 86 (2015) 8–23 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Multi-locus fossil-calibrated phylogeny of Atheriniformes (Teleostei, Ovalentaria) Daniela Campanella a, Lily C. Hughes a, Peter J. Unmack b, Devin D. Bloom c, Kyle R. Piller d, ⇑ Guillermo Ortí a, a Department of Biological Sciences, The George Washington University, Washington, DC, USA b Institute for Applied Ecology, University of Canberra, Australia c Department of Biology, Willamette University, Salem, OR, USA d Department of Biological Sciences, Southeastern Louisiana University, Hammond, LA, USA article info abstract Article history: Phylogenetic relationships among families within the order Atheriniformes have been difficult to resolve Received 29 December 2014 on the basis of morphological evidence. Molecular studies so far have been fragmentary and based on a Revised 21 February 2015 small number taxa and loci. In this study, we provide a new phylogenetic hypothesis based on sequence Accepted 2 March 2015 data collected for eight molecular markers for a representative sample of 103 atheriniform species, cover- Available online 10 March 2015 ing 2/3 of the genera in this order. The phylogeny is calibrated with six carefully chosen fossil taxa to pro- vide an explicit timeframe for the diversification of this group. Our results support the subdivision of Keywords: Atheriniformes into two suborders (Atherinopsoidei and Atherinoidei), the nesting of Notocheirinae Silverside fishes within Atherinopsidae, and the monophyly of tribe Menidiini, among others. We propose taxonomic Marine to freshwater transitions Marine dispersal changes for Atherinopsoidei, but a few weakly supported nodes in our phylogeny suggests that further Molecular markers study is necessary to support a revised taxonomy of Atherinoidei. -

SPECIAL SCIENTIFIC REPORT-FISHERIES Na 631
A UNITED STATES DEPARTMENT OF COMMERCE PUBLICATION U.S. DEPARTMENT OF COMMERCE NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION NATIONAL MARINE FISHERIES SERVICE Occurrence of Thiaminase in Some Common Aquatic Animals of the United States and Canada 1971 SPECIAL SCIENTIFIC REPORT-FISHERIES Na 631 UNITED STATES DEPARTMENT OF COMMERCE Maurice H. Stans, Secretary NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION Dr. Robert M. White, Administrator NATIONAL MARINE FISHERIES SERVICE Philip M. Roedel, Director Occurrence of Thiaminase in Some Common Aquatic Animals of the United States and Canada By R. A. GREIG and R. H. GNAEDINGER Special Scientific Report—Fisheries No. 631 Seattle, Washington July 1971 CONTENTS Page Introduction 1 Explanation of the tables 2 Discussion 2 Literature cited 3 TABLES 1. Thiaminase presence in freshwater animals 4 2. Thiaminase presence in marine animals 6 in Occurrence of Thiaminase in Some Common Aquatic Animals of the United States and Canada By R. A. GREIG National Marine Fisheries Service Technological Laboratory, Ann Arbor, Michigan 48107 and R. H. GNAEDINGER Pet Food Nutritional Research, Ralston-Purina Company, Checkerboard Square, St. Louis, Missouri 63199 ABSTRACT Two tables are presented that survey the presence or absence of thiaminase in freshwater and marine fish and shellfish. INTRODUCTION vitally important to animal feeders, particu- larly mink ranchers, for safety and economic The presence of thiaminase in fish that are reasons. routinely used raw in rations for animals can Also, scientific researchers at times need to cause a dietary deficiency. The disease in mink consider whether or not an aquatic animal is commonly called Chastek paralysis (Green, involved in their research contains thiaminase.