BULLETIN of the ALLYN MUSEUM 3621 Bayshore Rd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

January 1990
TORREYANA Published for Members of the Torrey Pines Docent Society and the Torrey Pines Association No. 172 January 1990 Next Docent Society Meeting SATURDAY, JANUA..t<¥ 20, 9: 00 A.M. AT THE VISI'IDR CENI'ER David F. Marriott will speak on "Butterflies of the TPSR Area" at the January meeting (alsc> see his article in this nonth 1 s Torreyana). He will show specimens of all species, ·as ...e11 as slides, photos, videos, and rredia coverage. Dr. Marriott is currently teaching a course through UCSD Extension on the butterflies of San Diego County, with an emphasis on rbnarch migration. He has been studying the butterfly species of the TPSR area during the past five years, and has studied and collected nearly all the ~ : sp:cies from San Diego County. For information requested for the business part of the meeting, see the Docent President 1 s notes below . ...~·~ .· Docent President's Notes by Michael K. Fox What a great_Christrnas party! (pictures~ p. 2) On behalf of the docents and directors of t.~ Torrey Pines Docent Society, I wish to extend cor71plirnents and thanks to the class of 1 89 for a job well done. Thanks to each and every one of :you! Since the new year is UPJrl us, it is tirre to do a little retrospective analysis ··-you know, hin£1~~ight. For the January rreeting, the Board of Directors v..ould like to have suggestJ:'6fi~ from the general rrembership for nolT'inations for "Docent of the Year." Please .. either :r;ail your choice to rre or bring it on a 3x5 card to hand in at the rreeting. -

Natural Communities of Michigan: Classification and Description
Natural Communities of Michigan: Classification and Description Prepared by: Michael A. Kost, Dennis A. Albert, Joshua G. Cohen, Bradford S. Slaughter, Rebecca K. Schillo, Christopher R. Weber, and Kim A. Chapman Michigan Natural Features Inventory P.O. Box 13036 Lansing, MI 48901-3036 For: Michigan Department of Natural Resources Wildlife Division and Forest, Mineral and Fire Management Division September 30, 2007 Report Number 2007-21 Version 1.2 Last Updated: July 9, 2010 Suggested Citation: Kost, M.A., D.A. Albert, J.G. Cohen, B.S. Slaughter, R.K. Schillo, C.R. Weber, and K.A. Chapman. 2007. Natural Communities of Michigan: Classification and Description. Michigan Natural Features Inventory, Report Number 2007-21, Lansing, MI. 314 pp. Copyright 2007 Michigan State University Board of Trustees. Michigan State University Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover photos: Top left, Dry Sand Prairie at Indian Lake, Newaygo County (M. Kost); top right, Limestone Bedrock Lakeshore, Summer Island, Delta County (J. Cohen); lower left, Muskeg, Luce County (J. Cohen); and lower right, Mesic Northern Forest as a matrix natural community, Porcupine Mountains Wilderness State Park, Ontonagon County (M. Kost). Acknowledgements We thank the Michigan Department of Natural Resources Wildlife Division and Forest, Mineral, and Fire Management Division for funding this effort to classify and describe the natural communities of Michigan. This work relied heavily on data collected by many present and former Michigan Natural Features Inventory (MNFI) field scientists and collaborators, including members of the Michigan Natural Areas Council. -

Bibliographic Guide to the Terrestrial Arthropods of Michigan
The Great Lakes Entomologist Volume 16 Number 3 - Fall 1983 Number 3 - Fall 1983 Article 5 October 1983 Bibliographic Guide to the Terrestrial Arthropods of Michigan Mark F. O'Brien The University of Michigan Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation O'Brien, Mark F. 1983. "Bibliographic Guide to the Terrestrial Arthropods of Michigan," The Great Lakes Entomologist, vol 16 (3) Available at: https://scholar.valpo.edu/tgle/vol16/iss3/5 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. O'Brien: Bibliographic Guide to the Terrestrial Arthropods of Michigan 1983 THE GREAT LAKES ENTOMOLOGIST 87 BIBLIOGRAPHIC GUIDE TO THE TERRESTRIAL ARTHROPODS OF MICHIGAN Mark F. O'Brienl ABSTRACT Papers dealing with distribution, faunal extensions, and identification of Michigan insects and other terrestrial arthropods are listed by order, and cover the period of 1878 through 1982. The following bibliography lists the publications dealing with the distribution or identification of insects and other terrestrial arthropods occurring in the State of Michigan. Papers dealing only with biological, behavioral, or economic aspects are not included. The entries are grouped by orders, which are arranged alphabetically, rather than phylogenetic ally , to facilitate information retrieval. The intent of this paper is to provide a ready reference to works on the Michigan fauna, although some of the papers cited will be useful for other states in the Great Lakes region. -

Annotated Checklist of the Butterflies of Bentsen-Rio Grande Valley State
AN ANNOTATED CHECKLIST OF THE BUTTERFLIES (LEPIDOPTERA: RHOPALOCERA) OF BENTSEN-RIO GRANDE STATE VALLEY PARK AND VICINITY JUNE, 1974 Published by TEXAS PARKS & WILDLIFE DEPARTMENT BENTSEN-RIO GRANDE VALLEY STATE PARK P.O. 30X 988; MISSION, TEXAS 78572 INTRODUCTION The species listed here in are primarily a result of the collecting by the authors during the period 1972-1973. Certain important records of the previous several years are also included. Additionally, the checklist incorporates records of a number of other lepidopterists. The primary focus of the checklist, then, is upon recent collecting, rather than being an attempt to list all known records from the Mid-Valley area. All lepidopterists collecting in the park and vicinity are urged to send copies of their records to the authors and/or the park authorities. A number of species on the list have been taken in Hidalgo Co. but not yet within the actual confines of the park; the annotations will indicate which species these are. Some of these have been taken at Santa Ana National Wildlife Refuge, approximately thirty miles down river, in habitats similar to those within the park. Others have been taken within several miles of the park, in nearby towns and along roadsides. These species can be reasonably expected to occur in the park, and their inclusion upon this list should alert the collector to their possible presence. The annotations have been kept necessarily brief. They are intended to aid the visiting lepidopterist in evaluating the significance of his catches. Local larval food plants are given where known. Much, however, is still to be learned regarding the life histories of even some of the commoner species. -
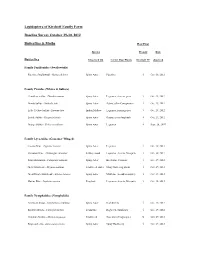
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Specimen Records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895
Catalog: Oregon State Arthropod Collection 2019 Vol 3(2) Specimen records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895 Jon H. Shepard Paul C. Hammond Christopher J. Marshall Oregon State Arthropod Collection, Department of Integrative Biology, Oregon State University, Corvallis OR 97331 Cite this work, including the attached dataset, as: Shepard, J. S, P. C. Hammond, C. J. Marshall. 2019. Specimen records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895. Catalog: Oregon State Arthropod Collection 3(2). (beta version). http://dx.doi.org/10.5399/osu/cat_osac.3.2.4594 Introduction These records were generated using funds from the LepNet project (Seltmann) - a national effort to create digital records for North American Lepidoptera. The dataset published herein contains the label data for all North American specimens of Lycaenidae and Riodinidae residing at the Oregon State Arthropod Collection as of March 2019. A beta version of these data records will be made available on the OSAC server (http://osac.oregonstate.edu/IPT) at the time of this publication. The beta version will be replaced in the near future with an official release (version 1.0), which will be archived as a supplemental file to this paper. Methods Basic digitization protocols and metadata standards can be found in (Shepard et al. 2018). Identifications were confirmed by Jon Shepard and Paul Hammond prior to digitization. Nomenclature follows that of (Pelham 2008). Results The holdings in these two families are extensive. Combined, they make up 25,743 specimens (24,598 Lycanidae and 1145 Riodinidae). -

Guide for Constructed Wetlands
A Maintenance Guide for Constructed of the Southern WetlandsCoastal Plain Cover The constructed wetland featured on the cover was designed and photographed by Verdant Enterprises. Photographs Photographs in this books were taken by Christa Frangiamore Hayes, unless otherwise noted. Illustrations Illustrations for this publication were taken from the works of early naturalists and illustrators exploring the fauna and flora of the Southeast. Legacy of Abundance We have in our keeping a legacy of abundant, beautiful, and healthy natural communities. Human habitat often closely borders important natural wetland communities, and the way that we use these spaces—whether it’s a back yard or a public park—can reflect, celebrate, and protect nearby natural landscapes. Plant your garden to support this biologically rich region, and let native plant communities and ecologies inspire your landscape. A Maintenance Guide for Constructed of the Southern WetlandsCoastal Plain Thomas Angell Christa F. Hayes Katherine Perry 2015 Acknowledgments Our thanks to the following for their support of this wetland management guide: National Oceanic and Atmospheric Administration (grant award #NA14NOS4190117), Georgia Department of Natural Resources (Coastal Resources and Wildlife Divisions), Coastal WildScapes, City of Midway, and Verdant Enterprises. Additionally, we would like to acknowledge The Nature Conservancy & The Orianne Society for their partnership. The statements, findings, conclusions, and recommendations are those of the author(s) and do not necessarily reflect the views of DNR, OCRM or NOAA. We would also like to thank the following professionals for their thoughtful input and review of this manual: Terrell Chipp Scott Coleman Sonny Emmert Tom Havens Jessica Higgins John Jensen Christi Lambert Eamonn Leonard Jan McKinnon Tara Merrill Jim Renner Dirk Stevenson Theresa Thom Lucy Thomas Jacob Thompson Mayor Clemontine F. -

Little Metalmark, Calephelis Virginiensis (Guérin- Ménéville) (Insecta: Lepidoptera: Riodinidae)1 Donald W
EENY-407 Little Metalmark, Calephelis virginiensis (Guérin- Ménéville) (Insecta: Lepidoptera: Riodinidae)1 Donald W. Hall, Jerry F. Butler, and Marc Minno2 Introduction The little metalmark, Calephelis virginiensis (Guérin- Ménéville), is one of three allopatric metalmarks found in the eastern United States. Although the little metalmark is one of our tiniest butterflies, it is one of our most beautiful. It is the only metalmark found in the southeastern coastal plain. Distribution Southeastern coastal plain from southeastern Virginia to eastern Texas in uplands and marginal wetlands, sandhills, flatwoods, pine savannas, prairies, and on roadsides. Figure 1. Adult little metalmark, Calephelis virginiensis (Guérin- Description Ménéville). Adults Credits: Jerry F. Butler, UF/IFAS The wings vary in color from rusty orange to orange-brown and have metallic silver lines on the wings (Figure 1)—the characteristic from which the family gets the common name “metalmarks”. The wingspan is 12–25 mm. Eggs The flattened eggs are reddish-brown with white sculptur- ing (Figure 2). Figure 2. Egg of the little metalmark, Calephelis virginiensis (Guérin- Ménéville). Credits: Jerry F. Butler, UF/IFAS 1. This document is EENY-407, one of a series of the Entomology and Nematology Department, UF/IFAS Extension. Original publication date May 2007. Revised December 2016. Reviewed October 2019. Visit the EDIS website at https://edis.ifas.ufl.edu for the currently supported version of this publication. This document is also available on the Featured Creatures website at http://entomology.ifas.ufl.edu/creatures. 2. Donald W. Hall and Jerry F. Butler, Entomology and Nematology Department, UF/IFAS Extension, Gainesville, FL; and Marc Minno, Suwannee River Water Management District. -

Final Lower Rio Grande Valley and Santa Ana National Wildlife
Final Lower Rio Grande Valley and Santa Ana National Wildlife Refuges Comprehensive Conservation Plan September 1997 (Reprint March 1999) U.S. Fish and Wildlife Service U.S. Department of the Interior Cover Artwork by Brian Cobble Table of Contents VISION........................................................................................................................................... 5 Executive Summary................................................................................................................... 6 1.0 Introduction and Regional Setting................................................................................. 8 1.1 LRGV Challenges............................................................................................... 8 2.0 Planning Perspectives and Considerations................................................................ 9 2.1 National Wildlife Refuge System ................................................................... 9 2.2 The Service & Ecosystem Management ...................................................... 9 2.3 Refuge Complex and Management Districts........................................... 10 2.4 Laguna Atascosa NWR -- A Partner with LRGV NWR............................ 10 2.5 Planning Perspectives.................................................................................... 10 2.6 The Issues.......................................................................................................... 11 2.7 The Need for Action........................................................................................ -

Checklist of Texas Lepidoptera Knudson & Bordelon, Jan 2018 Texas Lepidoptera Survey
1 Checklist of Texas Lepidoptera Knudson & Bordelon, Jan 2018 Texas Lepidoptera Survey ERIOCRANIOIDEA TISCHERIOIDEA ERIOCRANIIDAE TISCHERIIDAE Dyseriocrania griseocapitella (Wlsm.) Eriocraniella mediabulla Davis Coptotriche citripennella (Clem.) Eriocraniella platyptera Davis Coptotriche concolor (Zell.) Coptotriche purinosella (Cham.) Coptotriche clemensella (Cham). Coptotriche sulphurea (F&B) NEPTICULOIDEA Coptotriche zelleriella (Clem.) Tischeria quercitella Clem. NEPTICULIDAE Coptotriche malifoliella (Clem.) Coptotriche crataegifoliae (Braun) Ectoedemia platanella (Clem.) Coptotriche roseticola (F&B) Ectoedemia rubifoliella (Clem.) Coptotriche aenea (F&B) Ectoedemia ulmella (Braun) Asterotriche solidaginifoliella (Clem.) Ectoedemia obrutella (Zell.) Asterotriche heliopsisella (Cham.) Ectoedemia grandisella (Cham.) Asterotriche ambrosiaeella (Cham.) Nepticula macrocarpae Free. Asterotriche helianthi (F&B) Stigmella scintillans (Braun) Asterotriche heteroterae (F&B) Stigmella rhoifoliella (Braun) Asterotriche longeciliata (F&B) Stigmella rhamnicola (Braun) Asterotriche omissa (Braun) Stigmella villosella (Clem.) Asterotriche pulvella (Cham.) Stigmella apicialbella (Cham.) Stigmella populetorum (F&B) Stigmella saginella (Clem.) INCURVARIOIDEA Stigmella nigriverticella (Cham.) Stigmella flavipedella (Braun) PRODOXIDAE Stigmella ostryaefoliella (Clem.) Stigmella myricafoliella (Busck) Tegeticula yuccasella (Riley) Stigmella juglandifoliella (Clem.) Tegeticula baccatella Pellmyr Stigmella unifasciella (Cham.) Tegeticula carnerosanella Pellmyr -

Arizona Wildlife Notebook
ARIZONA WILDLIFE CONSERVATION ARIZONA WILDLIFE NOTEBOOK GARRY ROGERS Praise for Arizona Wildlife Notebook “Arizona Wildlife Notebook” by Garry Rogers is a comprehensive checklist of wildlife species existing in the State of Arizona. This notebook provides a brief description for each of eleven (11) groups of wildlife, conservation status of all extant species within that group in Arizona, alphabetical listing of species by common name, scientific names, and room for notes. “The Notebook is a statewide checklist, intended for use by wildlife watchers all over the state. As various individuals keep track of their personal observations of wildlife in their specific locality, the result will be a more selective checklist specific to that locale. Such information would be vitally useful to the State Wildlife Conservation Department, as well as to other local agencies and private wildlife watching groups. “This is a very well-documented snapshot of the status of wildlife species – from bugs to bats – in the State of Arizona. Much of it should be relevant to neighboring states, as well, with a bit of fine-tuning to accommodate additions and deletions to the list. “As a retired Wildlife Biologist, I have to say Rogers’ book is perhaps the simplest to understand, yet most comprehensive in terms of factual information, that I have ever had occasion to peruse. This book should become the default checklist for Arizona’s various state, federal and local conservation agencies, and the basis for developing accurate local inventories by private enthusiasts as well as public agencies. "Arizona Wildlife Notebook" provides a superb starting point for neighboring states who may wish to emulate Garry Rogers’ excellent handiwork. -

Book Reviews, New Publica- Tions, Metamorphosis, Announcements
________________________________________________________________________________________ Volume 57, Number 4 Winter 2015 www.lepsoc.org ________________________________________________________________________________________ Inside: Butterflies of Bolivia Are there caterpillars on butterfly wings? A citizen science call for action Ghost moths of the world website A conservation concern from the 1870’s Fruit-feeding Nymphali- dae in a west Mexican neotropical garden Fender’s Blue Butterfly conservation and re- covery Membership Updates, Marketplace, Book Reviews, New Publica- tions, Metamorphosis, Announcements ... ... and more! ________________________________________________________________________________________ ________________________________________________________ Contents ________________________________________________________www.lepsoc.org Species diversity and temporal distribution in a community of fruit- ____________________________________ feeding Nymphalidae in a west Mexican neotropical garden Volume 57, Number 4 Gerald E. Einem and William Adkins. ............................................... 163 Winter 2015 Windows for butterfly nets The Lepidopterists’ Society is a non-profit ed- J. Alan Wagar. ................................................................................... 173 ucational and scientific organization. The ob- Announcements: .......................................................................................... 174 ject of the Society, which was formed in May Zone Coordinator Needed; Season Summary