Action Potential and Bursting Phenomena Using Analog Electrical Neuron
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interactions Among Diameter, Myelination, and the Na/K Pump Affect Axonal Resilience to High-Frequency Spiking
Interactions among diameter, myelination, and the Na/K pump affect axonal resilience to high-frequency spiking Yunliang Zanga,b and Eve Mardera,b,1 aVolen National Center for Complex Systems, Brandeis University, Waltham, MA 02454; and bDepartment of Biology, Brandeis University, Waltham, MA 02454 Contributed by Eve Marder, June 22, 2021 (sent for review March 25, 2021; reviewed by Carmen C. Canavier and Steven A. Prescott) Axons reliably conduct action potentials between neurons and/or (16–21). It remains unknown how these directly correlated pro- other targets. Axons have widely variable diameters and can be cesses, fast spiking and slow Na+ removal, interact in the control myelinated or unmyelinated. Although the effect of these factors of reliable spike generation and propagation. Furthermore, it is on propagation speed is well studied, how they constrain axonal unclear whether reducing Na+-andK+-current overlap can con- resilience to high-frequency spiking is incompletely understood. sistently decrease the rate of Na+ influx and accordingly enhance Maximal firing frequencies range from ∼1Hzto>300 Hz across axonal reliability to propagate high-frequency spikes. Conse- neurons, but the process by which Na/K pumps counteract Na+ quently, by modeling the process of Na+ removal in unmyelinated influx is slow, and the extent to which slow Na+ removal is com- and myelinated axons, we systematically explored the effects of patible with high-frequency spiking is unclear. Modeling the pro- diameter, myelination, and Na/K pump density on spike propagation cess of Na+ removal shows that large-diameter axons are more reliability. resilient to high-frequency spikes than are small-diameter axons, because of their slow Na+ accumulation. -

Energy Expenditure Computation of a Single Bursting Neuron
Cognitive Neurodynamics (2019) 13:75–87 https://doi.org/10.1007/s11571-018-9503-3 (0123456789().,-volV)(0123456789().,-volV) ORIGINAL ARTICLE Energy expenditure computation of a single bursting neuron 2 1,2 2 2 Fengyun Zhu • Rubin Wang • Xiaochuan Pan • Zhenyu Zhu Received: 17 October 2017 / Revised: 22 August 2018 / Accepted: 28 August 2018 / Published online: 3 September 2018 Ó The Author(s) 2018 Abstract Brief bursts of high-frequency spikes are a common firing pattern of neurons. The cellular mechanisms of bursting and its biological significance remain a matter of debate. Focusing on the energy aspect, this paper proposes a neural energy calculation method based on the Chay model of bursting. The flow of ions across the membrane of the bursting neuron with or without current stimulation and its power which contributes to the change of the transmembrane electrical potential energy are analyzed here in detail. We find that during the depolarization of spikes in bursting this power becomes negative, which was also discovered in previous research with another energy model. We also find that the neuron’s energy consumption during bursting is minimal. Especially in the spontaneous state without stimulation, the total energy con- sumption (2.152 9 10-7 J) during 30 s of bursting is very similar to the biological energy consumption (2.468 9 10-7 J) during the generation of a single action potential, as shown in Wang et al. (Neural Plast 2017, 2017a). Our results suggest that this property of low energy consumption could simply be the consequence of the biophysics of generating bursts, which is consistent with the principle of energy minimization. -

Spiking Neural Networks: Neuron Models, Plasticity, and Graph Applications
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2015 Spiking Neural Networks: Neuron Models, Plasticity, and Graph Applications Shaun Donachy Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Theory and Algorithms Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/3984 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. c Shaun Donachy, July 2015 All Rights Reserved. SPIKING NEURAL NETWORKS: NEURON MODELS, PLASTICITY, AND GRAPH APPLICATIONS A Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science at Virginia Commonwealth University. by SHAUN DONACHY B.S. Computer Science, Virginia Commonwealth University, May 2013 Director: Krzysztof J. Cios, Professor and Chair, Department of Computer Science Virginia Commonwealth University Richmond, Virginia July, 2015 TABLE OF CONTENTS Chapter Page Table of Contents :::::::::::::::::::::::::::::::: i List of Figures :::::::::::::::::::::::::::::::::: ii Abstract ::::::::::::::::::::::::::::::::::::: v 1 Introduction ::::::::::::::::::::::::::::::::: 1 2 Models of a Single Neuron ::::::::::::::::::::::::: 3 2.1 McCulloch-Pitts Model . 3 2.2 Hodgkin-Huxley Model . 4 2.3 Integrate and Fire Model . 6 2.4 Izhikevich Model . 8 3 Neural Coding Techniques :::::::::::::::::::::::::: 14 3.1 Input Encoding . 14 3.1.1 Grandmother Cell and Distributed Representations . 14 3.1.2 Rate Coding . 15 3.1.3 Sine Wave Encoding . 15 3.1.4 Spike Density Encoding . 15 3.1.5 Temporal Encoding . 16 3.1.6 Synaptic Propagation Delay Encoding . -

Neuron C Reference Guide Iii • Introduction to the LONWORKS Platform (078-0391-01A)
Neuron C Provides reference info for writing programs using the Reference Guide Neuron C programming language. 078-0140-01G Echelon, LONWORKS, LONMARK, NodeBuilder, LonTalk, Neuron, 3120, 3150, ShortStack, LonMaker, and the Echelon logo are trademarks of Echelon Corporation that may be registered in the United States and other countries. Other brand and product names are trademarks or registered trademarks of their respective holders. Neuron Chips and other OEM Products were not designed for use in equipment or systems, which involve danger to human health or safety, or a risk of property damage and Echelon assumes no responsibility or liability for use of the Neuron Chips in such applications. Parts manufactured by vendors other than Echelon and referenced in this document have been described for illustrative purposes only, and may not have been tested by Echelon. It is the responsibility of the customer to determine the suitability of these parts for each application. ECHELON MAKES AND YOU RECEIVE NO WARRANTIES OR CONDITIONS, EXPRESS, IMPLIED, STATUTORY OR IN ANY COMMUNICATION WITH YOU, AND ECHELON SPECIFICALLY DISCLAIMS ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of Echelon Corporation. Printed in the United States of America. Copyright © 2006, 2014 Echelon Corporation. Echelon Corporation www.echelon.com Welcome This manual describes the Neuron® C Version 2.3 programming language. It is a companion piece to the Neuron C Programmer's Guide. -

Characteristics of Hippocampal Primed Burst Potentiation in Vitro and in the Awake Rat
The Journal of Neuroscience, November 1988, 8(11): 4079-4088 Characteristics of Hippocampal Primed Burst Potentiation in vitro and in the Awake Rat D. M. Diamond, T. V. Dunwiddie, and G. M. Rose Medical Research Service, Veterans Administration Medical Center, and Department of Pharmacology, University of Colorado Health Sciences Center, Denver, Colorado 80220 A pattern of electrical stimulation based on 2 prominent (McNaughton, 1983; Lynch and Baudry, 1984; Farley and Al- physiological features of the hippocampus, complex spike kon, 1985; Byrne, 1987). Of these, a phenomenontermed long- discharge and theta rhythm, was used to induce lasting in- term potentiation (LTP) has received extensive attention be- creases in responses recorded in area CA1 of hippocampal causeit sharesa number of characteristicswith memory. For slices maintained in vitro and from the hippocampus of be- example, LTP has a rapid onset and long duration and is having rats. This effect, termed primed burst (PB) potentia- strengthenedby repetition (Barnes, 1979; Teyler and Discenna, tion, was elicited by as few as 3 stimuli delivered to the 1987). In addition, the decay of LTP is correlated with the time commissural/associational afferents to CAl. The patterns courseof behaviorally assessedforgetting (Barnes, 1979; Barnes of stimulus presentation consisted of a single priming pulse and McNaughton, 1985). The similarity of LTP and memory followed either 140 or 170 msec later by a high-frequency has fostered an extensive characterization of the conditions un- burst of 2-10 pulses; control stimulation composed of un- derlying the initiation of LTP, as well as its biochemical sub- primed high-frequency trains of up to 10 pulses had no en- strates (Lynch and Baudry, 1984; Wigstrijm and Gustafsson, during effect. -

The Logic Behind Neural Control of Breathing Pattern Alona Ben-Tal 1, Yunjiao Wang2 & Maria C
www.nature.com/scientificreports OPEN The logic behind neural control of breathing pattern Alona Ben-Tal 1, Yunjiao Wang2 & Maria C. A. Leite3 The respiratory rhythm generator is spectacular in its ability to support a wide range of activities and Received: 10 January 2019 adapt to changing environmental conditions, yet its operating mechanisms remain elusive. We show Accepted: 29 May 2019 how selective control of inspiration and expiration times can be achieved in a new representation of Published: xx xx xxxx the neural system (called a Boolean network). The new framework enables us to predict the behavior of neural networks based on properties of neurons, not their values. Hence, it reveals the logic behind the neural mechanisms that control the breathing pattern. Our network mimics many features seen in the respiratory network such as the transition from a 3-phase to 2-phase to 1-phase rhythm, providing novel insights and new testable predictions. Te mechanism for generating and controlling the breathing pattern by the respiratory neural circuit has been debated for some time1–6. In 1991, an area of the brainstem, the pre Bötzinger Complex (preBötC), was found essential for breathing7. An isolated single PreBötC neuron could generate tonic spiking (a non-interrupted sequence of action potentials), bursting (a repeating pattern that consists of a sequence of action potentials fol- lowed by a time interval with no action potentials) or silence (no action potentials)8. Tese signals are transmit- ted, through other populations of neurons, to spinal motor neurons that activate the respiratory muscle4,6. Te respiratory muscle contracts when it receives a sequence of action potentials from the motor neurons and relaxes when no action potentials arrive9. -

Scripting NEURON
Scripting NEURON Robert A. McDougal Yale School of Medicine 7 August 2018 What is a script? A script is a file with computer-readable instructions for performing a task. In NEURON, scripts can: set-up a model, define and perform an experimental protocol, record data, . Why write scripts for NEURON? Automation ensures consistency and reduces manual effort. Facilitates comparing the suitability of different models. Facilitates repeated experiments on the same model with different parameters (e.g. drug dosages). Facilitates recollecting data after change in experimental protocol. Provides a complete, reproducible version of the experimental protocol. Programmer's Reference neuron.yale.edu Use the \Switch to HOC" link in the upper-right corner of every page if you need documentation for HOC, NEURON's original programming language. HOC may be used in combination with Python: use h.load file to load a HOC library; the functions and classes are then available with an h. prefix. Introduction to Python Displaying results The print command is used to display non-graphical results. It can display fixed text: print ('Hello everyone.') Hello everyone. or the results of a calculation: print (5 * (3 + 2)) 25 Storing results Give values a name to be able to use them later. a = max([1.2, 5.2, 1.7, 3.6]) print (a) 5.2 In Python 2.x, print is a keyword and the parentheses are unnecessary. Using the parentheses allows your code to work with both Python 2.x and 3.x. Don't repeat yourself Lists and for loops To do the same thing to several items, put the items in a list and use a for loop: numbers = [1, 3, 5, 7, 9] for number in numbers: print (number * number) 1 9 25 49 81 Items can be accessed directly using the [] notation; e.g. -

NEST by Example: an Introduction to the Neural Simulation Tool NEST Version 2.6.0
NEST by Example: An Introduction to the Neural Simulation Tool NEST Version 2.6.0 Marc-Oliver Gewaltig1, Abigail Morrison2, and Hans Ekkehard Plesser3, 2 1Blue Brain Project, Ecole Polytechnique Federale de Lausanne, QI-J, Lausanne 1015, Switzerland 2Institute of Neuroscience and Medicine (INM-6) Functional Neural Circuits Group, J¨ulich Research Center, 52425 J¨ulich, Germany 3Dept of Mathematical Sciences and Technology, Norwegian University of Life Sciences, PO Box 5003, 1432 Aas, Norway Abstract The neural simulation tool NEST can simulate small to very large networks of point-neurons or neurons with a few compartments. In this chapter, we show by example how models are programmed and simulated in NEST. This document is based on a preprint version of Gewaltig et al. (2012) and has been updated for NEST 2.6 (r11744). Updated to 2.6.0 Hans E. Plesser, December 2014 Updated to 2.4.0 Hans E. Plesser, June 2014 Updated to 2.2.2 Hans E. Plesser & Marc-Oliver Gewaltig, December 2012 1 Introduction NEST is a simulator for networks of point neurons, that is, neuron models that collapse the morphol- ogy (geometry) of dendrites, axons, and somata into either a single compartment or a small number of compartments (Gewaltig and Diesmann, 2007). This simplification is useful for questions about the dynamics of large neuronal networks with complex connectivity. In this text, we give a practical intro- duction to neural simulations with NEST. We describe how network models are defined and simulated, how simulations can be run in parallel, using multiple cores or computer clusters, and how parts of a model can be randomized. -

NEURON and Python
ORIGINAL RESEARCH ARTICLE published: 28 January 2009 NEUROINFORMATICS doi: 10.3389/neuro.11.001.2009 NEURON and Python Michael L. Hines1, Andrew P. Davison2* and Eilif Muller3 1 Computer Science, Yale University, New Haven, CT, USA 2 Unité de Neurosciences Intégratives et Computationelles, CNRS, Gif sur Yvette, France 3 Laboratory for Computational Neuroscience, Ecole Polytechnique Fédérale de Lausanne, Switzerland Edited by: The NEURON simulation program now allows Python to be used, alone or in combination with Rolf Kötter, Radboud University, NEURON’s traditional Hoc interpreter. Adding Python to NEURON has the immediate benefi t Nijmegen, The Netherlands of making available a very extensive suite of analysis tools written for engineering and science. Reviewed by: Felix Schürmann, Ecole Polytechnique It also catalyzes NEURON software development by offering users a modern programming Fédérale de Lausanne, Switzerland tool that is recognized for its fl exibility and power to create and maintain complex programs. At Volker Steuber, University of the same time, nothing is lost because all existing models written in Hoc, including graphical Hertfordshire, UK user interface tools, continue to work without change and are also available within the Python Arnd Roth, University College London, UK context. An example of the benefi ts of Python availability is the use of the xml module in *Correspondence: implementing NEURON’s Import3D and CellBuild tools to read MorphML and NeuroML model Andrew Davison, UNIC, Bât. 32/33, specifi cations. CNRS, 1 Avenue de la Terrasse, 91198 Keywords: Python, simulation environment, computational neuroscience Gif sur Yvette, France. e-mail: [email protected] INTRODUCTION for the purely numerical issue of how many compartments are The NEURON simulation environment has become widely used used to represent each of the cable sections. -

NEURAL CONNECTIONS: Some You Use, Some You Lose
NEURAL CONNECTIONS: Some You Use, Some You Lose by JOHN T. BRUER SOURCE: Phi Delta Kappan 81 no4 264-77 D 1999 . The magazine publisher is the copyright holder of this article and it is reproduced with permission. Further reproduction of this article in violation of the copyright is prohibited JOHN T. BRUER is president of the James S. McDonnell Foundation, St. Louis. This article is adapted from his new book, The Myth of the First Three Years (Free Press, 1999), and is reprinted by arrangement with The Free Press, a division of Simon Schuster Inc. ©1999, John T. Bruer . OVER 20 YEARS AGO, neuroscientists discovered that humans and other animals experience a rapid increase in brain connectivity -- an exuberant burst of synapse formation -- early in development. They have studied this process most carefully in the brain's outer layer, or cortex, which is essentially our gray matter. In these studies, neuroscientists have documented that over our life spans the number of synapses per unit area or unit volume of cortical tissue changes, as does the number of synapses per neuron. Neuroscientists refer to the number of synapses per unit of cortical tissue as the brain's synaptic density. Over our lifetimes, our brain's synaptic density changes in an interesting, patterned way. This pattern of synaptic change and what it might mean is the first neurobiological strand of the Myth of the First Three Years. (The second strand of the Myth deals with the notion of critical periods, and the third takes up the matter of enriched, or complex, environments.) Popular discussions of the new brain science trade heavily on what happens to synapses during infancy and childhood. -
The Action Potential
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/6316219 The action potential ARTICLE in PRACTICAL NEUROLOGY · JULY 2007 Source: PubMed CITATIONS READS 16 64 2 AUTHORS, INCLUDING: Mark W Barnett The University of Edinburgh 21 PUBLICATIONS 661 CITATIONS SEE PROFILE Available from: Mark W Barnett Retrieved on: 24 October 2015 192 Practical Neurology HOW TO UNDERSTAND IT Pract Neurol 2007; 7: 192–197 The action potential Mark W Barnett, Philip M Larkman t is over 60 years since Hodgkin and called ion channels that form the permeation Huxley1 made the first direct recording of pathways across the neuronal membrane. the electrical changes across the neuro- Although the first electrophysiological nal membrane that mediate the action recordings from individual ion channels were I 2 potential. Using an electrode placed inside a not made until the mid 1970s, Hodgkin and squid giant axon they were able to measure a Huxley predicted many of the properties now transmembrane potential of around 260 mV known to be key components of their inside relative to outside, under resting function: ion selectivity, the electrical basis conditions (this is called the resting mem- of voltage-sensitivity and, importantly, a brane potential). The action potential is a mechanism for quickly closing down the transient (,1 millisecond) reversal in the permeability pathways to ensure that the polarity of this transmembrane potential action potential only moves along the axon in which then moves from its point -
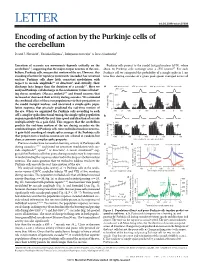
(2015) Encoding of Action by the Purkinje Cells of the Cerebellum
LETTER doi:10.1038/nature15693 Encoding of action by the Purkinje cells of the cerebellum David J. Herzfeld1, Yoshiko Kojima2, Robijanto Soetedjo2 & Reza Shadmehr1 Execution of accurate eye movements depends critically on the Purkinje cells project to the caudal fastigial nucleus (cFN), where cerebellum1–3, suggesting that the major output neurons of the cere- about 50 Purkinje cells converge onto a cFN neuron17. For each bellum, Purkinje cells, may predict motion of the eye. However, this Purkinje cell we computed the probability of a simple spike in 1-ms encoding of action for rapid eye movements (saccades) has remained time bins during saccades of a given peak speed, averaged across all unclear: Purkinje cells show little consistent modulation with respect to saccade amplitude4,5 or direction4, and critically, their discharge lasts longer than the duration of a saccade6,7.Herewe a 400° per s saccade 650° per s saccade 400° per s saccade 650° per s saccade analysed Purkinje-cell discharge in the oculomotor vermis of behav- 500° per s 8,9 200 ing rhesus monkeys (Macaca mulatta) and found neurons that N12 increased or decreased their activity during saccades. We estimated the combined effect of these two populations via their projections to 100 the caudal fastigial nucleus, and uncovered a simple-spike popu- lation response that precisely predicted the real-time motion of 0 the eye. When we organized the Purkinje cells according to each –1000 100 0 100 0 100 0 100 cell’s complex-spike directional tuning, the simple-spike population b 500° per s response predicted both the real-time speed and direction of saccade multiplicatively via a gain field.