A Simulation Model for Interaction Between Cyclic Variation of Brain Tissue Temperature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

School of Engineering and Technology, Apeejay Stya University
School of Engineering and Technology, Apeejay Stya University Gurgaon An exclusive Guide by School of Engineering and Technology, Apeejay Stya University Reviews on Placements, Faculty & Facilities Check latest reviews and ratings on placements, faculty, facilities submitted by students & alumni. Reviews (Showing 12 of 12 reviews) Overall Rating (Out of 5) 3.6 Based o n 11 Verif ied Reviews Distribution of Rating >4-5 star 27% >3-4 star 45% >2-3 star 27% 1-2 star 0% Component Ratings (Out of 5) Placements 3.5 Infrastructure 3.7 Faculty & Course 3.6 Curriculum Crowd & Campus Life 3.9 Value for Money 3.4 The Verif ied badge indicates that the reviewer's details have been verified by Shiksha, and reviewers are bona f ide students of this college. These reviews and ratings have been given by students. Shiksha does not endorsed the same. Out of 12 published reviews, 11 reviews are verif ied. F Faizan Ahmed | B.Tech. in Computer Science and Engineering - Batch of Verified 2021 Reviewed on 11 Jan 2020 3.2 Placements 3 Infrastructure 3 Faculty & Course Curriculum 4 Crowd & Campus Life 3 I am satisfied only with the faculty members. Placements: About 70% of students in our course got placed. The highest salary package offered is 20 LPA, and the average salary package offered is 3 LPA, and the lowest salary Disclaimer: This PDF is auto-generated based on the information available on Shiksha as on 30-Sep-2021. package offered is 1 LPA. Top recruiting companies like CarDekho, Wipro, etc., visit our campus for placements. -
CSR July 2018 Table Overall Ranking (F4).Pmd
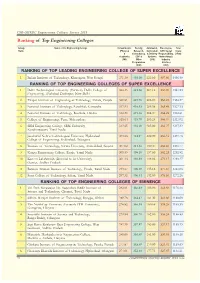
CSR-GHRDC Engineering Colleges Survey 2018 Ranking of Top Engineering Colleges Group Name of the Engineering College Infrastructure Faculty, Admission, Placements, Total Rank (Physical Research, Curriculum USP, Social Score & Consultancy, & Delivery Responsibility, (1700) Academic) EDP & Systems Networking & (390) Other (250) Industry Programmes Interface (600) (460) RANKING OF TOP LEADING ENGINEERING COLLEGE OF SUPER EXCELLENCE 1 Indian Institute of Technology, Kharagpur, West Bengal 371.38 454.30 223.00 407.80 1456.48 RANKING OF TOP ENGINEERING COLLEGES OF SUPER EXCELLENCE 1 Delhi Technological University (Formerly Delhi College of 324.15 423.56 207.18 387.05 1341.93 Engineering), Shahabad Daulatpur, New Delhi 2 Thapar Institute of Engineering & Technology, Patiala, Punjab 320.81 421.78 206.00 386.28 1334.87 3 National Institute of Technology, Surathkal, Karnataka 317.53 418.53 205.58 385.49 1327.13 4 National Institute of Technology, Rourkela, Odisha 314.39 419.16 204.97 384.29 1322.81 5 College of Engineering, Pune, Maharashtra 315.64 415.70 203.24 386.44 1321.02 6 SRM Engineering College, SRM University, 312.65 416.90 205.00 383.27 1317.82 Kancheepuram, Tamil Nadu 7 Jawaharlal Nehru Technological University Hyderabad, 309.28 413.97 202.00 382.51 1307.76 College of Engineering, Hyderabad, Telangana 8 Institute of Technology, Nirma University, Ahmedabad, Gujarat 307.02 411.52 199.37 383.85 1301.77 9 Kongu Engineering College, Erode, Tamil Nadu 305.49 408.10 197.00 382.23 1292.82 10 Koneru Lakshmaiah (Deemed to be University), 301.14 410.89 198.06 379.17 1289.27 Guntur, Andhra Pradesh 11 Bannari Amman Institute of Technology, Erode, Tamil Nadu 299.27 408.80 195.13 377.87 1281.06 12 Sona College of Technology, Salem, Tamil Nadu 297.32 406.44 192.00 376.50 1272.26 RANKING OF TOP ENGINEERING COLLEGES OF EMINENCE 1 Vel Tech Rangarajan Dr. -
UNIVERSITY GRANTS COMMISSION State-Wise List of Private
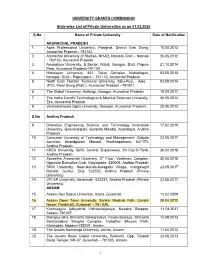
UNIVERSITY GRANTS COMMISSION State-wise List of Private Universities as on 01.02.2020 S.No Name of Private University Date of Notification ARUNACHAL PRADESH 1. Apex Professional University, Pasighat, District East Siang, 10.05.2013 Arunachal Pradesh - 791102. 2. Arunachal University of Studies, NH-52, Namsai, Distt – Namsai 26.05.2012 - 792103, Arunachal Pradesh. 3. Arunodaya University, E-Sector, Nirjuli, Itanagar, Distt. Papum 21.10.2014 Pare, Arunachal Pradesh-791109 4. Himalayan University, 401, Takar Complex, Naharlagun, 03.05.2013 Itanagar, Distt – Papumpare – 791110, Arunachal Pradesh. 5. North East Frontier Technical University, Sibu-Puyi, Aalo 03.09.2014 (PO), West Siang (Distt.), Arunachal Pradesh –791001. 6. The Global University, Hollongi, Itanagar, Arunachal Pradesh. 18.09.2017 7. The Indira Gandhi Technological & Medical Sciences University, 26.05.2012 Ziro, Arunachal Pradesh. 8. Venkateshwara Open University, Itanagar, Arunachal Pradesh. 20.06.2012 S.No Andhra Pradesh 9. Bharatiya Engineering Science and Technology Innovation 17.02.2019 University, Gownivaripalli, Gorantla Mandal, Anantapur, Andhra Pradesh 10. Centurian University of Technology and Management, Gidijala 23.05.2017 Junction, Anandpuram Mandal, Visakhapatnam- 531173, Andhra Pradesh. 11. KREA University, 5655, Central, Expressway, Sri City-517646, 30.04.2018 Andhra Pradesh 12. Saveetha Amaravati University, 3rd Floor, Vaishnavi Complex, 30.04.2018 Opposite Executive Club, Vijayawada- 520008, Andhra Pradesh 13. SRM University, Neerukonda-Kuragallu Village, mangalagiri 23.05.2017 Mandal, Guntur, Dist- 522502, Andhra Pradesh (Private University) 14. VIT-AP University, Amaravati- 522237, Andhra Pradesh (Private 23.05.2017 University) ASSAM 15. Assam Don Bosco University, Azara, Guwahati 12.02.2009 16. Assam Down Town University, Sankar Madhab Path, Gandhi 29.04.2010 Nagar, Panikhaiti, Guwahati – 781 036. -

Best Private Universities Ranking 2021
EDUCATION POST | May-June 2021 | 110 IIRF-2021| BEST PRIVATE UNIVERSITIES tate ame City Zone S N Rank* tate Rank Zone Rank Zone S Preferential Preferential Dhirubhai Ambani Institute of 1 Information and Communication Gandhinagar Gujarat 1 West 1 Technology 2 AMITY University Noida Uttar Pradesh 1 North 1 3 Shiv Nadar University Dadri Uttar Pradesh 2 North 2 4 O.P. Jindal Global University Sonipat Haryana 1 North 3 5 Nirma University Ahmedabad Gujarat 2 West 2 6 Reva University Bangalore Karnataka 1 South 1 7 Azim Premji University Bangalore Karnataka 2 South 2 8 Sri Sri University Bhubaneswar Odisha 1 East 1 9 AMITY University Jaipur Rajasthan 1 North 4 10 ITM University Gwalior Madhya Pradesh 1 Central 1 10 AMITY University Gwalior Madhya Pradesh 1 Central 1 11 Dayanand Sagar University Bangalore Karnataka 3 South 3 12 AMITY University Gurgaon Haryana 2 North 5 Centre for Environmental Planning Ahemdabad Gujarat West 13 and Technology University 3 3 13 Apeejay Stya University Sohna Haryana 3 North 6 14 Chitkara University Patiala Punjab 1 North 7 15 Ahmedabad University Ahmedabad Gujarat 4 West 4 Shoolini University of Biotechnology Solan Himachal Pradesh North 16 and Management Sciences 1 8 *Page 6 (Disclaimer) EDUCATION POST | May-June 2021 | 112 EDUCATION POST | May-June 2021 | 113 IIRF-2021| BEST PRIVATE UNIVERSITIES tate ame City Zone S N Rank* tate Rank Zone Rank Zone S Preferential Preferential University of Petroleum and Energy Dehradun Uttarakhand North 17 Studies (UPES) 1 9 18 Chandigarh University Mohali Punjab 2 North 10 -

Apeejay Stya University Is Located on the Sohna-Palwal Road in District Gurgaon of Haryana
Apeejay Stya University is located on the Sohna-Palwal Road in district Gurgaon of Haryana. The nearest railway station is Palwal which is approximately 18 kms away from the campus. Local trains and buses are available to and from Palwal. The University can be reached from Delhi via Faridabad-Palwal, as well as via Gurgaon, Rajeev Chowk and Sohna. ASU provides its students with bus facility from key pick-up points in Delhi, Faridabad & Gurgaon: Route 1 Route 2 Route 3 Starting from Malviya Nagar, New Delhi via Starting from Sector-4, 9, Starting from Sikanderpur Metro Ashram, Faridabad (Main Mathura Road) Rajeev Chowk via Sohna Station via Sec 55-56, Sohna For admissions and other queries, you can either visit the ASU Campus at Sohna, Apeejay School Campus, Sheikh Sarai, the ASU City Office at Gurgaon or any other Apeejay Institution t e l APEEJAY STYA ASU Campus: Apeejay Stya University, Sohna-Palwal Road, Sohna, k (South of South Delhi), Gurgaon, Haryana – 122103 o UNIVERSITY o Recognised by UGC ASU City Office: Apeejay Stya University Admissions Office, Plot No. 23, Sector 32, B Institutional Area, Gurgaon, Haryana -122001 ST n INDIA'S 1 Apeejay School Campus, Sheikh Sarai-Phase 1, Panchsheel Park, New Delhi - 110017 o i LIBERAL ARTS & t Admission counsellors of ASU can be contacted at : a META UNIVERSITY E-mail : [email protected] m FOCUSED ON r Toll Free Number: 1800-103-7888 o & f RESEARCH Landline: +91-124-4286870/1/2/3/4 | Mob: 8527735552, 9650473222 n I TECHNOLOGY AY EJ ST E Y P A 45+ A university.apeejay.edu DISCOVER THE APEEJAY STYA UNIVERSITY EDGE Carrying forth the Apeejay legacy of over 45 Years of excellence in education WE VALUE Commitment to Research ASU provides research opportunities across all levels including real-time industry-led research. -

NORTH ZONE 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 North Vc@Bbau
NORTH ZONE 01.04.2014 Dr. Taj Pratap Vice Chancellor A P G (Alakh Prakash Goyal) Shimla University Shimla 171009 North [email protected]; 1 1.4.2016 Prof A S Guleria Vice Chancellor Abhilashi University, Chailchowk, Tehsil Chachyot Dist Mandi 175 North [email protected]; 2 028 16.12.2016 Col. Dr G P I Singh Vice Chancellor Adesh University, Bhatinda 151 001 Punjab North [email protected]; 3 4 17.6.2016 Prof Anil Kumar Vice chancellor Al-FALAH University, Dhauj, Faridabad 121 004, Haryana North [email protected]; 5 01.04.1924 Prof Tariq Mansoor Vice chancellor Aligarh Muslim University, Aligarh 202 002 UP North [email protected]; 22.02.2019 Prof Anu Singh Lather Vice Chancellor Ambedkar University Delhi. Lothian Road, Kashmere Gate, New North [email protected]; 6 Delhi 110006 12.10.2010 Prof Balvinder Shukla Vice Chancellor Amity University Uttar Pradesh, Sector 125, NOIDA 201 313 District North [email protected]; 7 Gautam Budha Nagar 26.11.2013 Prof Dr P B Sharma Vice Chancellor Amity University, Amity Education Valley, Panchgaon, Manesar, North [email protected]; 8 Gurgaon dist 122 413 1.4.2018 Prof Raj Singh Vice Chancellor Ansal University, Sohna, Gururam North [email protected]; 9 01.04.2014 Dr S K Salwan Vice Chancellor Apeejay Stya University, Sohna Palwal Road, , Village Silani, North [email protected]; 10 Gurgaon 122 103 Haryana 11 26.11.2013 Brig S C Verma Vice Chancellor Arni University, Kathgarh, Tehsil Indora, District Kangar 176 401 HP North [email protected]; Prof Manoj K. -

Download Brochure
Apeejay Stya University India’s 1st Industry-Centric Technology & Liberal Arts UGC Recognised University focused on Research & Innovation™ What i need is a pair of wings wings of inspiration wings of encouragement wings of ambition wings of ideas wings of knowledge Freedom to choose your own majors & minors Carrying forth the Apeejay Stya legacy of over 50 years of excellence in education 1 WE VALUE WE PROVIDE WE FOCUS ON Commitment to Research Innovative Academic System Relevant Curriculum & Syllabus ASU provides research opportunities across all levels Based on the best practices of the Ivy League The focus is on well-designed curriculum that including real-time industry-led research. universities of USA. engages students and promotes critical thinking, creativity and achievement at all levels. Each course is Freedom to Realign Educational Dynamic Evaluation Procedure carefully selected & vetted, and is revisited regularly in sync with the changing market dynamics based on a Goals Continuous and dynamic evaluation system is ‘mark-to-market’ approach. A student can choose to change his/her degree or provided, including field projects, group discussions, research credits and take-home papers. major even in later semesters and re-apply credits Flexible Academic Load already gained. Unique Technology Driven With a flexible credit system, students can choose to take between 12 to 18 credits per semester and are Freedom to Graduate at One’s Teaching Methodology permitted to take a range of advanced classes. Pace Students and teachers at ASU discover knowledge ASU allows students to graduate at their own pace and skills together. Small classes & well-equipped Excellence in Research & (early or late) by choosing their own customized path. -
CSR BOX FINAL.Cdr
|TABLE OF CONTENTS Abbreviations Introduction i. Why is there a need for SDG-University linkages? ii. Why do SDGs need Universities? iii. What is the importance of such linkages for universities? iv. What can Universities do? SDG-Universities linkages in India and a brief snapshot of the world Analytics and Key Findings (in Haryana) i. Total Number of Universities researched ii. Spatial Distribution of all the Universities and Research Centers/Projects iii. Spatial Distribution of Universities having Research Centers/Projects iv. SDGs covered by the Public University's Research Centers/Projects v. SDGs covered by the Private University's Research Centers/Projects vi. Spatial Distribution of Public and Private Universities vii. Total number of SDGs covered. by the research centers/projects Key SDGs covered by the Research Centres/Projects i. Zero Hunger SDG ii. Good Health and Well Being iii. Quality Education iv. Gender Equality v. Clean Water and Sanitation Case Studies Way Forward Annexure 2 |ABBREVIATIONS AICTE All India Council for Technical Education ASERF Apeejay Stya Education Research Foundation CAFT Centre of Advanced Faculty Training CAWTM Center for Advanced Water Technology & Management CECRAS Center for Excellence in Corporate Responsibility and Sustainability CEEES The Center of Excellence for Energy and Environmental Studies CESH Center for Environment, Sustainability and Human Development CGHEF Centre For Global Higher Education Futures CSBC Centre for Social and Behaviour Change CSEAS Center For Southeast Asian Studies -

Apeejay Stya Education Research Foundation 10
Bulletin No.88 Date: 16th July - 31st July 2010 Announcements ASERF has instituted Dr Stya Paul Young CONTENT Educationists Award’ for honouring Young Educationists who have demonstrated their potential Aspect by making an impact on Indian education. Entry of foreign universities, whom does it benefit? Applications from the eligible scholars are invited for the Award of the year 2010. Click here to download News the prescribed format along with the terms and conditions. 1. New institutes worried about AICTE decision Apeejay Education Society announces the 2. As schools log in to digital learning, on-line assessment the rage 3. School heads' delegation visits University of Malta opening of Apeejay Stya University 4. State to get database on technical education The Apeejay Education Society, with over 40 5. Kalam launches ‘Save Our Daughters India' movement..... years of Excellence in education, announces 6. State plans law to govern CBSE, ICSE schools the initial opening of Apeejay Stya University at 7. Clueless on number of seats, state stalls tech admissions Sohna, Gurgaon. Sponsored by the Apeejay Stya Education Foundation, the University is 8. Draft law unveiled for ‘Innovation Universities' currently located in a sprawling picturesque 9. Innovation universities can have foreign Vice-Chancellors, says draft Bill campus with the state-of-the-art infrastructure. 10. DU colleges put students ahead of UGC's norms for ad hoc teachers Apeejay Stya University will offer a diverse 11. New course on intellectual property rights catalogue of technical, scientific, management 12. Super Fast 'Science and Education Internet' for India by August and liberal arts courses for the academic 13. -

Apeejay Stya Education Research Foundation (ASERF) Invites News, Articles, Resource Material, 22
Bulletin No.109 Date: 1st June - 15th June 2011 Announcements CONTENT ASERF has instituted Dr Stya Paul Young Educationist Award’ for honouring Young Educationists who have Aspect demonstrated their potential by making an impact on Indian education. Applications from the eligible scholars are invited for the Cloud computing in education Award of the year 2011. Click here to download the prescribed format along with the terms and conditions. Apeejay Stya University announces admission News for the session 2011-12 1. Battle between AICTE, institutes over PGDM courses continues Apeejay Stya University is offering diverse catalogue of technical, scientific, management and liberal arts courses 2. Number of overseas students continue to fall in Australia for the academic session 2011-12. Applicants for admission 3. State seeks AICTE approval for 52 new technical institutes accepted on the basis of comprehensive merit, judged by 4. HRD Ministry proposes to extend RTE till Class 10 their academic excellence, their extracurricular achievements, and their utilization of the resources they 5. Pearson Education Seeks Larger Presence in India have had available. As part of the application, the University 6. Shri Kapil Sibal Addresses 58th Meeting of CABE recognize a number of examination scores to establish academic excellence, including AIEEE, GMAT, SAT, and 7. Summary Record of Discussions of the 58th CABE SAT II. 8. Discussion of the State Education Ministers’ Conference For more, visit: www.apeejay.edu/asu 9. Education summit in Canada Apeejay Signs MOU with Dutch and French 10. Twin engineering degree Universities Analysis/Opinion/Innovative Practice Apeejay Institute of Design (AID), New Delhi and Apeejay Stya University (ASU), Haryana signed (MOU) with AKV| St. -

Data of Sexual Harassment Updated Till JANUARY 2020 (3)
A list of cases of sexual harassment in higher education institutions during one year i.e. 01/04/2018 to 31/03/2019 (updated upto November,2019) (state‐wise and College/Institute‐wise) : S. No. State Name of the university Number of Number of Number of Number of workshops on Nature of Internal Complaint cases of sexual Complaints cases pending Awareness Programmes action Committee harassment disposed off for more than against sexual constituted or not received during the 90 days harassment conducted during01/04/20 year during the year 18 to 31/03/2019 Gujarat 1 Navrachana University, Nil Nil Nil 2 Nil Yes Vadodara 2 Veer Narmad South Nil Nil Nil Nil Nil No Gujrat University 3 Nirma University Nil Nil Nil 14 Nil No Ahmedabad 4 UKA Tarsadia University, Nil Nil Nil 5 Nil Nil Dist. Surat, Gujarat 5 Gokul Global University Nil Nil Nil 3 Nil Yes 6 Sardar Patel University Nil Nil Nil 1 Nil yes 7 Department of sociology Nil Nil Nil Nil Nil Nil sadar patel university , 8 Ahmedabad University Nil Nil Nil 11 Nil Yes 9 Atmiya University Nil Nil Nil Nil Workshops Yes conducted on 30 april 26‐ 27 Dec 2018 and 22‐23 Jan 20189 10 Gujarat National Law 3 3 Nil 2 Respondant Yes University, Gandhinagar was releived from all his duties and responsibilitie s from GNLU campus Respondant suspended from the Halls of residence 11 Central University of Nil Nil Nil 1 Nil Yes Gujarat Uttar Pradesh 12 Sharda University, Noida 8 8 Nil 6 Case‐I : Yes (Ref:SU/Reg./ Order/ 2018/ 598) 1. -

University Grants Commission
UNIVERSITY GRANTS COMMISSION Total No. of Universities in the Country as on 22.02.2017 Universities Total No. State Universities 359 Deemed to be Universities 123 Central Universities 47 Private Universities 260 Total 789 1 S.No ANDHRA PRADESH Date/Year of Notification/ Establishment 1. Acharya Nagarjuna University, Nagarjuna Nagar-522510, Dt. Guntur, Andhra 1976 Pradesh. (State University) 2. Adikavi Nannaya University, 25-7-9/1, Jayakrishnapuram, Rajahmundry – 533 2006 105, East Godavari District, Andhra Pradesh. (State University) 3. Andhra University, Waltair, Visakhapatnam-530 003, Andhra Pradesh. (State 1926 University) 4. Damodaram Sanjivayya National Law University, Plot No. 116, Sector 11 MVP 2008 Colony, Visakhapatnam – 530 017, Andhra Pradesh. (State University) 5. Dr. B.R. Ambedkar University, Etcherla, Dt. Srikakulam-532410, Andhra 2008 Pradesh. (State University) 6. Dravidian University, Srinivasanam, -517 425, Chittoor District, Andhra 1997 Pradesh. (State University) 7. Dr. Y.S.R. Horticultural University, PO Box No. 7, Venkataramannagudem, 2011 West Godavari District – 536 101, Andhra Pradesh. (State University) 8. Dr. N.T.R. University of Health Sciences (Formerly Andhra Pradesh University 1986 of Health Sciences), Vijayawada-520 008, Andhra Pradesh. (State University) 9. Gandhi Institute of Technology and Management (GITAM), Gandhi Nagar 13.08.2007 Campus, Rushikonda, Visakhapatman – 530 045, Andhra Pradesh.(Deemed University) 10. Jawaharlal Nehru Technological University, Anantpur-515 002, Andhra 2008 Pradesh (State University) 11. Jawaharlal Nehru Technological University, Pithapuram Road, Kakinada- 2008 533003, East Godvari District, Andhra Pradesh.(State University) 12. Koneru Lakshmaiah Education Foundation, Greenfields, Kunchanapalli Post, 20.02.2009 Vaddeswaram, Guntur District-522002, Andhra Pradesh. (Deemed University) 13. Krishna University, Andhra Jateeya Kalasala, Campus, Rajupeta, 2008 Machllipatanam – 521 001, Krishna District, Andhra Pradesh.