The Jellification of North Temperate Lakes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Literature Review of the Effects of Persistent Toxic Substanceson Great Lakes Biota
Renort to the Literature Review of the Effects of Persistent Toxic Substanceson Great Lakes Biota Report of the Health of Aquatic Communities Task Force by J. Fitchko IEC Beak Consultants Ltd. Mississauga, Ontario International Joint Commission Great Lakes Regional Office Windsor, Ontario December, 1986 TABLE OF CONTENTS Page PR EFACE V C 1.0 SUMMARY OF GREATLAKES RESEARCH 1 1.1 Summary of StructuralResponse Studies of GreatLakes Biota and Persistent Toxic Substances 1 1.2 Summary of FunctionalResponse Studies of GreatLakes Biota and Persistent Toxic Substances 1 1.3 Summary of HumanToxicology Studies Related to Persistent Toxic Substances in the Great Lakes 4 1.4 Synthesis of Researchon Effects of PersistentToxic Substances on Great Lakes Aquatic Populations 4 I. 5 Conclusions 12 2.0 INTRODUCTION 14 3.0 STUDYBACKGROUND AND APPROACH 25 3.1 DEAK'sStudy Approach 27 4.0 STIiIJCTURALINDICATORS OF HEALTH 34 4. I Rac ter ia 34 4.2 Phytoplankton 35 4.3 Periphyton 38 4.4 Macrophy ton 39 4.5 Zooplankton 41 4.6 Benthos 43 4.7 Fish 54 5.0 F'lJNCTIONALINDICATORS OF HEALTH 56 5. I PhysiologicalResponses 56 5.1.1 Bacteria ' 56 5.1.2 Phytoplankton 60 1 Page 5.1.3 Periphyton 70 5.1.4 Macrophyton 71 5.1.5 Zooplankton 72 5.1.6 Benthos 77 5.1.7 Fish 81 5.1.8 Amphibians 95 5. I. 9 Birds 96 5.2.10 Mammals 107 5.2 Behavioral Responses i10 5.2. I Zooplankton 110 5.2.2 Benthos 110 5.2.3 Fish 114 5.2.4 Amphibians 119 5.2.5 Birds 119 5.3 Genetic and Morphological Responses 121 5.3.1 MutagenicAssays 122 5.3.2 Phytoplankton 123 5.3.3 Benthos 124 3.3.4 Fish 128 5.3.5 -

Long-Term Trends in Lake-Wide Phytoplankton Productivity in the Upper Great Lakes: 1998-2013
Michigan Technological University Digital Commons @ Michigan Tech Michigan Tech Research Institute Publications Michigan Tech Research Institute 2015 Long-term trends in lake-wide phytoplankton productivity in the Upper Great Lakes: 1998-2013 Gary L. Fahnenstiel Michigan Technological University Michael J. Sayers Michigan Technological University Robert A. Shuchman Michigan Technological University S. A. Pothoven NOAA GLERL Foad Yousef Michigan Technological University Follow this and additional works at: https://digitalcommons.mtu.edu/mtri_p Part of the Physical Sciences and Mathematics Commons Recommended Citation Fahnenstiel, G. L., Sayers, M. J., Shuchman, R. A., Pothoven, S. A., & Yousef, F. (2015). Long-term trends in lake-wide phytoplankton productivity in the Upper Great Lakes: 1998-2013. IAGLR 58th Annual Conference on Great Lakes Research. Retrieved from: https://digitalcommons.mtu.edu/mtri_p/72 Follow this and additional works at: https://digitalcommons.mtu.edu/mtri_p Part of the Physical Sciences and Mathematics Commons ABSTRACTS International Association for Great Lakes Research ABSTRACTS 58th Annual Conference on Great Lakes Research May 25–29, 2015 University of Vermont © 2015 International Association for Great Lakes Research 4890 South State Road Ann Arbor, Michigan 48108 Cover design and conference logo by Jenifer Thomas CONTENTS ABSTRACTS .......................................................................................................... 1 A ....................................................................................................................... -

Effects of Calcium and Phosphorus Limitation on the Nutritional Ecophysiology of Daphnia
LIMNOLOGY and Limnol. Oceanogr. 61, 2016, 268–278 OCEANOGRAPHY VC 2015 Association for the Sciences of Limnology and Oceanography doi: 10.1002/lno.10208 Effects of calcium and phosphorus limitation on the nutritional ecophysiology of Daphnia Clay Prater†,*1 Nicole D. Wagner†,1 Paul C. Frost2 1Environmental and Life Science Graduate Program, Trent University, Peterborough, Ontario, Canada 2Department of Biology, Trent University, Peterborough, Ontario, Canada Abstract Declines in environmental calcium (Ca) and phosphorus (P) concentrations have occurred over the past 30 yrs in lakes across the Canadian Shield in southern Ontario, and these reductions appear to be placing strong constraints on populations of Daphnia in this region. Here, we report results from a factorial manipu- lation of Ca concentrations and food P content under controlled laboratory conditions where we measured resulting changes in daphnid elemental content, individual growth and survival, and life history traits related to population growth. We found significant effects of Ca- and P-limitation on all variables measured; how- ever, dietary P explained a majority of the variation in daphnid nutrient content and growth. Dietary effects of low P [high food carbon (C): P ratios] on individual Daphnia life-history traits also translated into signifi- cant population level effects. Dietary P also explained relatively more experimental variation in population level responses than Ca concentrations. Experimental Ca concentrations most strongly altered daphnid sur- vival partly due to the use of a lethally low Ca concentration in our experiment. Although recent work exam- ining shifts in zooplankton communities in this region mainly focuses on the effects of Ca-limitation, we show that Ca concentrations and food nutrient content, at levels commonly found on the Canadian Shield, are both likely to strongly alter Daphnia life-history and populations dynamics. -

Using Elemental Ratios of Calcium and Strontium to Track Calcium Availability in the Freshwater Zooplankton Daphnia Pulicaria Stephen C
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113, G04023, doi:10.1029/2008JG000782, 2008 Click Here for Full Article Using elemental ratios of calcium and strontium to track calcium availability in the freshwater zooplankton Daphnia pulicaria Stephen C. Peters,1 Ryan Lockwood,1 Craig E. Williamson,2 and Jasmine E. Saros3 Received 11 May 2008; revised 27 August 2008; accepted 23 September 2008; published 3 December 2008. [1] The assimilation of elements by an organism is dependent on both the environmental availability of the element and the processes of the organism. For some elements, organisms have a challenging time discriminating between nearly identical chemical analogs, for example, calcium and strontium. We tested the hypothesis that in environments where a desired element is scarce, the organism will assimilate a chemically similar analog at an increased rate. Populations of Daphnia pulicaria were manipulated using a microcosm in situ experiment and the results of that experiment tested with a field survey. Experimental results indicated a correlation between higher environmental calcium concentration and lower [Sr]/[Ca] ratios (R2 = 0.91, p < 0.05), suggesting that Daphnia in high calcium environments will assimilate more calcium relative to strontium. Field survey results across eight lakes confirmed that as lake calcium concentration increased, the value of [Sr]/[Ca] between the organism and the lake water decreased (R2 = 0.60, p < 0.05). Measurement of the elemental ratio of major and trace element analogs within organisms compared to their environments may be a useful tool for measuring the relative bioavailability of the major element, and provide insight into elemental limitation in other calcifying aquatic invertebrates. -

Zootaxa,Three New Cryptic Species Of
Zootaxa 1656: 1–49 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) Three new cryptic species of the freshwater zooplankton genus Holopedium (Crustacea: Branchiopoda: Ctenopoda), revealed by genetic methods CHAD L. ROWE1, SARAH J. ADAMOWICZ2 & PAUL D. N. HEBERT3,4 Biodiversity Institute of Ontario, Department of Integrative Biology, University of Guelph, Guelph, Ontario, N1G 2W1, Canada. 2Department of Biology, University of Waterloo, 200 University Avenue West, Waterloo, Ontario, N2L 3G1, Canada. E-mail: [email protected]; [email protected]; [email protected]; 4Corresponding author Table of contents Abstract ...............................................................................................................................................................................1 Introduction .........................................................................................................................................................................2 Methods ...............................................................................................................................................................................3 Results .................................................................................................................................................................................8 Overview and species identities ..........................................................................................................................................8 -

ERSS-Spiny Waterflea (Bythotrephes Longimanus)
Spiny Waterflea (Bythotrephes longimanus) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, February 2011 Revised, September 2014 and October 2016 Web Version, 11/29/2017 Photo: J. Liebig, NOAA Great Lakes Environmental Research Laboratory 1 Native Range, and Status in the United States Native Range From CABI (2016): “B. longimanus is native to the Baltic nations, Norway, northern Germany and the European Alps, covering Switzerland, Austria, Italy and southern Germany. It is also native to the British Isles and the Caucasus region, and is widespread in Russia.” Status in the United States From Liebig et al. (2016): “Bythotrephes is established in all of the Great Lakes and many inland lakes in the region. Densities are very low in Lake Ontario, low in southern Lake Michigan and offshore areas of Lake Superior, moderate to high in Lake Huron, and very high in the central basin of Lake Erie (Barbiero et al. 2001, Vanderploeg et al. 2002, Brown and Branstrator 2004).” 1 “Bythotrephes was first detected in December 1984 in Lake Huron (Bur et al. 1986), then Lake Ontario in September 1985 (Lange and Cap 1986), Lake Erie in October 1985 (Bur et al. 1986), Lake Michigan in September 1986 (Evans 1988), and Lake Superior in August 1987 (Cullis and Johnson 1988).” “Collected from Long Lake approximately 5 miles SW of Traverse City, Michigan (P. Marangelo, unpublished data). Established in Greenwood Lake and Flour Lake, Minnesota (D. Branstrator, pers. comm.). Collected in Allegheny Reservoir, New York (R. Hoskin, pers. comm.).” Means of Introductions in the United States From Liebig et al. -

Zootaxa,Three New Cryptic Species of the Freshwater
Zootaxa 1656: 1–49 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) Three new cryptic species of the freshwater zooplankton genus Holopedium (Crustacea: Branchiopoda: Ctenopoda), revealed by genetic methods CHAD L. ROWE1, SARAH J. ADAMOWICZ2 & PAUL D. N. HEBERT3,4 Biodiversity Institute of Ontario, Department of Integrative Biology, University of Guelph, Guelph, Ontario, N1G 2W1, Canada. 2Department of Biology, University of Waterloo, 200 University Avenue West, Waterloo, Ontario, N2L 3G1, Canada. E-mail: [email protected]; [email protected]; [email protected]; 4Corresponding author Table of contents Abstract ...............................................................................................................................................................................1 Introduction .........................................................................................................................................................................2 Methods ...............................................................................................................................................................................3 Results .................................................................................................................................................................................8 Overview and species identities ..........................................................................................................................................8 -

The Noncosmopolitanism Paradigm of Freshwater Zooplankton
Molecular Ecology (2009) 18, 5161–5179 doi: 10.1111/j.1365-294X.2009.04422.x The noncosmopolitanism paradigm of freshwater zooplankton: insights from the global phylogeography of the predatory cladoceran Polyphemus pediculus (Linnaeus, 1761) (Crustacea, Onychopoda) S. XU,* P. D. N. HEBERT,† A. A. KOTOV‡ and M. E. CRISTESCU* *Great Lakes Institute for Environmental Research, University of Windsor, Windsor, ON, Canada N9B 3P4, †Biodiversity Institute of Ontario, University of Guelph, Guelph, ON, Canada N1G 2W1, ‡A. N. Severtsov Institute of Ecology and Evolution, Leninsky Prospect 33, Moscow 119071, Russia Abstract A major question in our understanding of eukaryotic biodiversity is whether small bodied taxa have cosmopolitan distributions or consist of geographically localized cryptic taxa. Here, we explore the global phylogeography of the freshwater cladoceran Polyphemus pediculus (Linnaeus, 1761) (Crustacea, Onychopoda) using two mitochon- drial genes, cytochrome c oxidase subunit I and 16s ribosomal RNA, and one nuclear marker, 18s ribosomal RNA. The results of neighbour-joining and Bayesian phylogenetic analyses reveal an exceptionally pronounced genetic structure at both inter- and intra- continental scales. The presence of well-supported, deeply divergent phylogroups across the Holarctic suggests that P. pediculus represents an assemblage of at least nine, largely allopatric cryptic species. Interestingly, all phylogenetic analyses support the reciprocal paraphyly of Nearctic and Palaearctic clades. Bayesian inference of ancestral distribu- tions suggests that P. pediculus originated in North America or East Asia and that European lineages of Polyphemus were established by subsequent intercontinental dispersal events from North America. Japan and the Russian Far East harbour exceptionally high levels of genetic diversity at both regional and local scales. -

Comparison of Limnological Characteristics
National Park Service U.S. Department of the Interior Natural Resource Program Center Comparison of Limnological Characteristics and Distribution and Abundance of Littoral Macroinvertebrates and Zooplankton in Fishbearing and Fishless Lakes of Lassen Volcanic National Park Natural Resource Technical Report NPS/KLMN/NRTR—2008/116 ON THE COVER Lassen Peak at Sunset Photograph by: John Speece, Field Crew Member and Environmental Studies Student at Southern Oregon University __________________________________________________ Comparison of Limnological Characteristics and Distribution and Abundance of Littoral Macroinvertebrates and Zooplankton in Fishbearing and Fishless Lakes of Lassen Volcanic National Park Natural Resource Technical Report NPS/KLMN/NRTR—2008/116 Michael S. Parker Department of Biology Southern Oregon University Ashland, OR 97520 August 2008 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado i The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientific community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peerreviewed to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. The Natural Resources Technical Reports series is used to disseminate the peerreviewed results of scientific studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. The reports provide contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. -

Dependence of Long-Term Dynamics of Zooplankton in the Ob River on Interannual Changes in Hydrological and Hydrochemical Parameters
water Article Dependence of Long-Term Dynamics of Zooplankton in the Ob River on Interannual Changes in Hydrological and Hydrochemical Parameters Nadezhda Yermolaeva * , Serafima Dvurechenskaya, Vladimir Kirillov and Aleksandr Puzanov Institute for Water and Environmental Problems, Siberian Branch of the Russian Academy of Sciences, Molodezhnaya Str. 1, 656038 Barnaul, Russia; serafi[email protected] (S.D.); [email protected] (V.K.); [email protected] (A.P.) * Correspondence: [email protected]; Tel.: +7-952-903-11-59 Abstract: Here we summarize a long-term study on qualitative and quantitative composition of zooplankton (Cladocera, Copepoda, Rotifera) in the Ob River. We carried out these investigations at 13 sampling stations of the Middle and Low Ob in the years 1994, 1996, 1999, 2001, 2002 and 2009. It was found that the species richness of all zooplankton and abundance of cladocerans and rotifers is significantly determined by the temperature conditions of the month preceding sampling. In contrast, among other factors, we revealed that pH decreases as well as phosphate and nitrate concentrations increase zooplankton abundance. Dissolved oxygen and oxidizable organic substances (BOD ) were 5 positively correlated to copepod population levels (according to abundance and biomass); on the other hand, an increase in difficult-to-oxidize substances (COD) inhibited their development. During Citation: Yermolaeva, N.; Dvurechenskaya, S.; Kirillov, V.; this study, we found that high water levels had a positive influence on zooplankton richness in river Puzanov, A. Dependence of itself probably due to being downstream from Ob River floodplain lakes. Long-Term Dynamics of Zooplankton in the Ob River on Interannual Keywords: zooplankton; long-term dynamics; water level; Ob River; environmental factors Changes in Hydrological and Hydrochemical Parameters. -
Practical Guide to Identifying Freshwater Crustacean Zooplankton
Practical Guide to Identifying Freshwater Crustacean Zooplankton Cooperative Freshwater Ecology Unit 2004, 2nd edition Practical Guide to Identifying Freshwater Crustacean Zooplankton Lynne M. Witty Aquatic Invertebrate Taxonomist Cooperative Freshwater Ecology Unit Department of Biology, Laurentian University 935 Ramsey Lake Road Sudbury, Ontario, Canada P3E 2C6 http://coopunit.laurentian.ca Cooperative Freshwater Ecology Unit 2004, 2nd edition Cover page diagram credits Diagrams of Copepoda derived from: Smith, K. and C.H. Fernando. 1978. A guide to the freshwater calanoid and cyclopoid copepod Crustacea of Ontario. University of Waterloo, Department of Biology. Ser. No. 18. Diagram of Bosminidae derived from: Pennak, R.W. 1989. Freshwater invertebrates of the United States. Third edition. John Wiley and Sons, Inc., New York. Diagram of Daphniidae derived from: Balcer, M.D., N.L. Korda and S.I. Dodson. 1984. Zooplankton of the Great Lakes: A guide to the identification and ecology of the common crustacean species. The University of Wisconsin Press. Madison, Wisconsin. Diagrams of Chydoridae, Holopediidae, Leptodoridae, Macrothricidae, Polyphemidae, and Sididae derived from: Dodson, S.I. and D.G. Frey. 1991. Cladocera and other Branchiopoda. Pp. 723-786 in J.H. Thorp and A.P. Covich (eds.). Ecology and classification of North American freshwater invertebrates. Academic Press. San Diego. ii Acknowledgements Since the first edition of this manual was published in 2002, several changes have occurred within the field of freshwater zooplankton taxonomy. Many thanks go to Robert Girard of the Dorset Environmental Science Centre for keeping me apprised of these changes and for graciously putting up with my never ending list of questions. I would like to thank Julie Leduc for updating the list of zooplankton found within the Sudbury Region, depicted in Table 1. -
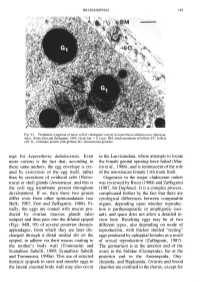
Ings for Leptestheria Dahalacensis. Even More Curious Is the Fact That
BRANCHIOPODA 151 Fig. 93. Peripheral cytoplasm of nurse cell of vitellogenic oocyte in Leptestheria dahalacensis (Spinicau- data). (From Zeni and Zaffagnini, 1989.) Scale bar — 0.5 \i.m. BM, basal membrane of follicle; FC, follicle cell; G|, immature protein yolk globule; IG, intracistemal granules. ings for Leptestheria dahalacensis. Even in the Laevicaudata, where attempts to locate more curious is the fact that, according to the female genital opening have failed (Mar these same authors, the egg envelope is cre tin et al., 1986), and is reminiscent of the role ated by exocytosis of the egg itself, rather of the notostracan female 11th trunk limb. than by secretions of oviducal cells (Notos- Oogenesis in the major cladoceran orders traca) or shell glands (Anostraca), and this is was reviewed by Rossi (1980) and Zaffagnini the only egg membrane present throughout (1987, for Daphnia). It is a complex process, development. If so, then these two genera complicated further by the fact that there are differ even from other spinicaudatans (see cytological differences between comparable Belk, 1987; Zeni and Zaffagnini, 1989). Fi organs, depending upon whether reproduc nally, the eggs are coated with mucus pro tion is parthenogenetic or amphigonic (sex duced by ovarian mucous glands (also ual), and space does not allow a detailed re unique) and then pass into the dilated epipod view here. Resulting eggs may be of two (Figs. 94B, 95) of several posterior thoracic different types, also depending on mode of appendages, from which they are later dis reproduction, with thicker shelled "resting" charged through a distal medial slit on the eggs produced by ephippial females as a result epipod, to adhere via their mucus coating to of sexual reproduction (Zaffagnini, 1987).