Guideline WHO Guidelines for Malaria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Neena Valecha1, Deepali Savargaonkar1, Bina Srivastava1, B
Valecha et al. Malar J (2016) 15:42 DOI 10.1186/s12936-016-1084-1 Malaria Journal RESEARCH Open Access Comparison of the safety and efficacy of fixed‑dose combination of arterolane maleate and piperaquine phosphate with chloroquine in acute, uncomplicated Plasmodium vivax malaria: a phase III, multicentric, open‑label study Neena Valecha1, Deepali Savargaonkar1, Bina Srivastava1, B. H. Krishnamoorthy Rao2, Santanu K. Tripathi3, Nithya Gogtay4, Sanjay Kumar Kochar5, Nalli Babu Vijaya Kumar6, Girish Chandra Rajadhyaksha7, Jitendra D. Lakhani8, Bhagirath B. Solanki9, Rajinder K. Jalali10, Sudershan Arora10, Arjun Roy10, Nilanjan Saha10, Sunil S. Iyer10, Pradeep Sharma10 and Anupkumar R. Anvikar1* Abstract Background: Chloroquine has been the treatment of choice for acute vivax malaria for more than 60 years. Malaria caused by Plasmodium vivax has recently shown resistance to chloroquine in some places. This study compared the efficacy and safety of fixed dose combination (FDC) of arterolane maleate and piperaquine phosphate (PQP) with chloroquine in the treatment of uncomplicated vivax malaria. Methods: Patients aged 13–65 years with confirmed mono-infection of P. vivax along with fever or fever in the previ- ous 48 h were included. The 317 eligible patients were randomly assigned to receive FDC of arterolane maleate and PQP (n 159) or chloroquine (n 158) for 3 days. Primaquine was given as an anti-relapse measure on day 3 and continued= for 14 consecutive days.= Primary efficacy analysis included assessment of the proportion of aparasitaemic and afebrile patients at 72 h. Safety endpoints were analysis of adverse events, vital signs, laboratory data, and abnor- malities on electrocardiograph. Patients participated in the study for at least 42 days. -

Trypanocidal Activity of Ethanolic Leaf Extract of Andrographis Paniculata
Science World Journal Vol. 15(No 4) 2020 www.scienceworldjournal.org ISSN: 1597-6343 (Online), ISSN: 2756-391X (Print) Published by Faculty of Science, Kaduna State University TRYPANOCIDAL ACTIVITY OF ETHANOLIC LEAF EXTRACT OF ANDROGRAPHIS PANICULATA 1* 2 3 4 Ladan Z., Olanrewaju T. O., Maikaje D.B., and Waziri P. N. Full Length Research Article 1Department of Chemistry, Kaduna State University, Kaduna, Nigeria 2Human African Trypanosomiasis Research Department, Nigerian Institute for Trypanosomiasis Research, Kaduna, Nigeria 3Department of Microbiology, Kaduna State University, Kaduna, Nigeria 4Department of Biochemistry, Kaduna State University, Kaduna, Nigeria *Corresponding Author’s Email Address: [email protected] Phone: 2347030475898 ABSTRACT animal Trypanosomiasis (Achenef and Bekele, 2013; Giordani et Andrographis paniculata used in this study belongs to Acanthacae al., 2016). Various industries are now on the search for sources of family and is commonly known as king of bitters. This study aimed alternative which include synthetic and natural products. Medicinal at evaluating effect of A. paniculata leaf extract on experimental plants remain a major source of alternative medicine and scientific rats infected with Trypanosoma brucei brucei. Cold maceration was investigations have shown that various plants indicate promising used for extraction and the biomass was fractionated in open results for the development of cheaper less toxic drugs for column chromatography. One of the ethanolic fractions obtained treatment and management of trypanosomiasis (Simoben et al., from A. paniculata was evaluated for in vitro and in vivo activities 2018). Andrographis paniculata evaluated in this study belong to using RPMI 1640 cell culture media and experimental wistar rats Acanthacae family of the plant kingdom and commonly known as respectively. -

Review Article Efforts Made to Eliminate Drug-Resistant Malaria and Its Challenges
Hindawi BioMed Research International Volume 2021, Article ID 5539544, 12 pages https://doi.org/10.1155/2021/5539544 Review Article Efforts Made to Eliminate Drug-Resistant Malaria and Its Challenges Wote Amelo 1,2,3 and Eyasu Makonnen 1,2 1Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, Addis Ababa University, Addis Ababa, Ethiopia 2Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), Addis Ababa University, Addis Ababa, Ethiopia 3Department of Pharmacology and Toxicology, School of Pharmacy, Jimma University, Jimma, Ethiopia Correspondence should be addressed to Wote Amelo; [email protected] Received 21 January 2021; Accepted 9 August 2021; Published 30 August 2021 Academic Editor: Jane Hanrahan Copyright © 2021 Wote Amelo and Eyasu Makonnen. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Since 2000, a good deal of progress has been made in malaria control. However, there is still an unacceptably high burden of the disease and numerous challenges limiting advancement towards its elimination and ultimate eradication. Among the challenges is the antimalarial drug resistance, which has been documented for almost all antimalarial drugs in current use. As a result, the malaria research community is working on the modification of existing treatments as well as the discovery and development of new drugs to counter the resistance challenges. To this effect, many products are in the pipeline and expected to be marketed soon. In addition to drug and vaccine development, mass drug administration (MDA) is under scientific scrutiny as an important strategy for effective utilization of the developed products. -

WHO Technical Consultation to Review the Role of Drugs in Malaria Prevention for People Living in Endemic Settings
Malaria Policy Advisory Committee Meeting 13—14 May 2020, Geneva, Switzerland Background document for Session 5 WHO technical consultation to review the role of drugs in malaria prevention for people living in endemic settings Meeting report, 16–17 October 2019, Geneva, Switzerland Summary On 16–17 October 2019, the World Health Organization (WHO) convened a Technical Consultation to review the use of medicines for malaria prevention in endemic countries and to identify opportunities to increase their impact through review of the flexibility of the recommendations for their deployment. Experts reviewed the policies and use of chemoprevention as currently endorsed by WHO, including intermittent preventive treatment in pregnancy (IPTp), intermittent preventive treatment in infants (IPTi), seasonal malaria chemoprevention (SMC) and mass drug administration (MDA) for the reduction of disease burden in emergency situations. By reviewing these strategies side-by-side for the first time, the meeting was able to consider opportunities for optimization. Additional potential applications of chemoprevention were also reviewed, and key considerations identified to develop a broader role for malaria chemoprevention in malaria-endemic populations. Key conclusions of the meeting included: • There is a need for general guidance on the broader use of chemoprevention. Chemoprevention is an important approach in the package of strategies that countries may use to decrease their malaria burden and/or move towards malaria elimination. Development of broader, more flexible guidance that builds on existing chemoprevention recommendations in specific populations is expected to support the greater, more rational use of chemoprevention and enhance its impact. • Chemoprevention strategies may be tailored to country-specific needs. Guidance should be flexible enough to enable adaptation of strategies according to the needs of different countries and settings. -

Update Tot 30-04-2020 1. Chloroquine and Hydroxychloroquine for The
Update tot 30-04-2020 1. Chloroquine and Hydroxychloroquine for the Prevention or Treatment of Novel Coronavirus Disease (COVID-19) in Africa: Caution for Inappropriate Off-Label Use in Healthcare Settings. Abena PM, Decloedt EH, Bottieau E, Suleman F, Adejumo P, Sam-Agudu NA, et al. Am j trop med hyg. 2020. 2. Evaluation of Hydroxychloroquine Retinopathy Using Ultra-Widefield Fundus Autofluorescence: Peripheral Findings in the Retinopathy. Ahn SJ, Joung J, Lee BR. American journal of ophthalmology. 2020;209:35-44. http://dx.doi.org/10.1016/j.ajo.2019.09.008. Epub 2019 Sep 14. 3. COVID-19 research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. Alexander PE, Debono VB, Mammen MJ, Iorio A, Aryal K, Deng D, et al. J clin epidemiol. 2020. 4. Chloroquine and Hydroxychloroquine in the Era of SARS - CoV2: Caution on Their Cardiac Toxicity. Bauman JL, Tisdale JE. Pharmacotherapy. 2020. 5. Repositioned chloroquine and hydroxychloroquine as antiviral prophylaxis for COVID-19: A protocol for rapid systematic review of randomized controlled trials. Chang R, Sun W-Z. medRxiv. 2020:2020.04.18.20071167. 6. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Colson P, Rolain J-M, Lagier J-C, Brouqui P, Raoult D. Int J Antimicrob Agents. 2020:105932-. 7. Dose selection of chloroquine phosphate for treatment of COVID-19 based on a physiologically based pharmacokinetic model. Cui C, Zhang M, Yao X, Tu S, Hou Z, Jie En VS, et al. Acta Pharmaceutica Sinica B. 2020. 8. Hydroxychloroquine; Why It Might Be Successful and Why It Might Not Be Successful in the Treatment of Covid-19 Pneumonia? Could It Be A Prophylactic Drug? Deniz O. -

Pharmaceutical Composition Comprising Artesunate
(19) TZZ ¥ 4B_T (11) EP 2 322 244 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61P 33/06 (2006.01) A61K 9/20 (2006.01) 21.11.2012 Bulletin 2012/47 A61K 31/357 (2006.01) A61K 31/505 (2006.01) A61K 31/4965 (2006.01) (21) Application number: 10192206.0 (22) Date of filing: 09.09.2005 (54) Pharmaceutical composition comprising artesunate, sulfamethoxypyrazine and pyrimethamine suitable for the treatment of malaria within one day, in the form of a tablet Pharmazeutische Zusammensetzung enthaltend Artesunat, Sulfamethoxypyrazin und Pyrimethamin, die zur Behandlung von Malaria innerhalb eines Tages geeignet ist, in Form einer Tablette Composition pharmaceutique à base d’artesunate, de sulfaméthoxypyrazine et de pyriméthamine permettant le traitement du palludisme en un jour, sous forme de comprimé (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • LUXEMBURGER C ET AL: "Single day HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI mefloquine-artesunate combination in the SK TR treatment of multi-drug resistant falciparum malaria", TRANSACTIONS OF THE ROYAL (43) Date of publication of application: SOCIETY OF TROPICAL MEDICINE AND 18.05.2011 Bulletin 2011/20 HYGIENE, ELSEVIER, GB, vol. 88, no. 2, 1 March 1994 (1994-03-01), pages 213-217, XP023097868, (62) Document number(s) of the earlier application(s) in ELSEVIER, GB ISSN: 0035-9203, DOI: accordance with Art. 76 EPC: 10.1016/0035-9203(94)90303-4 [retrieved on 05795966.0 / 1 940 519 1994-03-01] • DAO ET AL: "Fatty food does not alter blood (73) Proprietor: Dafra Pharma N.V. -

Meeting Report
Meeting Report EXPERT CONSULTATION ON PLASMODIUM KNOWLESI MALARIA TO GUIDE MALARIA ELIMINATION STRATEGIES 1–2 March 2017 Kota Kinabalu, Malaysia Expert Consultation on Plasmodium Knowlesi Malaria to Guide Malaria Elimination Strategies 1–2 March 2017 Kota Kinabalu, Malaysia WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC RS/2017/GE/05/(MYS) English only MEETING REPORT EXPERT CONSULTATION ON PLASMODIUM KNOWLESI MALARIA TO GUIDE MALARIA ELIMINATION STRATEGIES Convened by: WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC Kota Kinabalu, Malaysia 1–2 March 2017 Not for sale Printed and distributed by: World Health Organization Regional Office for the Western Pacific Manila, Philippines September 2017 NOTE The views expressed in this report are those of the participants of the Expert Consultation on Plasmodium knowlesi Malaria to Guide Malaria Elimination Strategies and do not necessarily reflect the policies of the World Health Organization. This report has been prepared by the World Health Organization Regional Office for the Western Pacific for governments of Member States in the Region and for those who participated in the Expert Consultation on Plasmodium knowlesi Malaria to Guide Malaria Elimination Strategies, which was held in Kota Kinabalu, Malaysia from 1 to 2 March 2017. CONTENTS ABBREVIATIONS SUMMARY 1. INTRODUCTION ............................................................................................................................................. 1 2. PROCEEDINGS ............................................................................................................................................... -
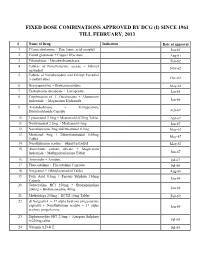
Fixed Dose Combinations Approved by Dcg (I) Since 1961 Till February, 2013
FIXED DOSE COMBINATIONS APPROVED BY DCG (I) SINCE 1961 TILL FEBRUARY, 2013 # Name of Drug Indication Date of approval 1. Cyanocobalamine + Zinc tannic acid complex Jan-61 2. Cobalt glutamate + Copper Glycinate Aug-61 3. Fibrinolysin + Desoxyribonuclease Feb-62 4. Tablets of Norethisterone acetate + Ethinyl Nov-62 oestradiol 5. Tablets of Norethynodrel and Ethinyl Estradiol 3-methyl ether Dec-62 6. Broxyquinoline + Brobenzoxalidine May-63 7. Testosterone decanoate + Isocaproate Jan-64 8. Combination of L Oxethazaine + Aluminium hydroxide + Magnesium Hydroxide Jun-66 9. Amylobarbitone + Trifluperazine Dihydrochloride Capsule Feb-67 10. Lynestronol 2.5mg + Mestranol 0.075mg Tablet Apr-67 11. Northynodrel 2.5mg + Mestranol 0.1mg Jun-67 12. Norethisterone 2mg and Mestranol 0.1mg May-67 13. Mestranol 4mg + Ethinyloestradial 0.05mg May-67 Tablet 14. Norethisterane acetate + ethinyl estradiol May-67 15. Aluminium sodium silicate + Magnesium hydroxide + Methypolysiloxane Tablet Jun-67 16. Ammoidin + Amidine Jul-67 17. Fluocortolene + Flucortolene Caproate Jul-68 18. Norgestrel + Ethinyloestradiol Tablet Aug-68 19. Folic Acid 0.5mg + Ferrous Sulphate 150mg Jan-69 Capsule 20. Tetracycline HCl 250mg + Broxyquinoline 200mg + Brobenzoxadine 40mg Jan-69 21. Methyldopa 250mg + HCTZ 15mg Tablet Feb-69 22. dl Norgestrel + 17 alpha hydroxy progesterone caproate + Norethisterone acetate + 17 alpha Jan-69 acetoxy progesterone 23. Diphenoxylate HCl 2.5mg + Atropine Sulphate 0.025mg tablet Jul-69 24. Vitamin A,D & E Jul-69 25. Lutin 0.1gm + Vit 0.1gm + Vit K1 2.5mg + Dicalcium Phosphate 0.1gm + Carlozochrome Jul-69 Salicylate 1mg tablet 26. Vit K 1 5mg + Calcium Lactolionate 100 m g+ Carlozocrome Salicylate 2.5mg + Phenol 0.5% Jul-69 + Lignocaine Hcl 1% injection 27. -

Analysis of the Effects of Malaria Chemoprophylaxis in Children on Haematological Responses, Morbidity and Mortality Paul D
Analysis of the effects of malaria chemoprophylaxis in children on haematological responses, morbidity and mortality Paul D. Prinsen Geerligs,1 Bernard J. Brabin,1,2 & Teunis A. Eggelte3 Abstract This paper reviews the evidence for beneficial effects of malaria chemoprophylaxis on haematological responses, morbidity, mortality, health service utilization and rebound immunity in children. As anaemia may play an important role in childhood mortality, it is important to assess evidence from controlled trials of the potential of chemoprophylaxis to reduce childhood anaemia. An analysis of trials found good evidence that malaria chemoprophylaxis improves mean haemoglobin levels and reduces severe anaemia, clinical malaria attacks, parasite and spleen rates. Significant reductions in outpatient attendance and hospital admissions have been achieved, and substantial evidence from Gambian studies shows reductions in mortality. Chemoprophylaxis in children does not seem to produce any sustained impairment of immunity to malaria, although rebound effects may be greater in children who receive prophylaxis during infancy. Short periods of targeted prophylaxis are likely to be preferable to continuous drug administration. Evidence of the protective efficacy of malaria chemoprophylaxis in children shows that this strategy could be considered within integrated health programmes for specific time periods. Intermittent routine combination therapy early in childhood may be appropriate for those living under holoendemic conditions. Large-scale studies over a number of years are needed to address this issue and the impact of this approach on health service utilization, mortality, and the emergence of drug-resistant parasites. Keywords Malaria/drug therapy/epidemiology; Malaria, Falciparum/drug therapy/epidemiology; Antimalarials/blood/therapeutic use; Anemia/drug therapy; Treatment outcome; Child; Infant mortality; Controlled clinical trials; Meta-analysis (source: MeSH, NLM). -

Infectious Disease Society of America Influenza Clinical Practice Guidelines
Clinical Infectious Diseases IDSA GUIDELINE Downloaded from https://academic.oup.com/cid/advance-article-abstract/doi/10.1093/cid/ciy866/5251935 by Massachusetts Public Health Department user on 21 December 2018 Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa Timothy M. Uyeki,1 Henry H. Bernstein,2 John S. Bradley,3,4 Janet A. Englund,5 Thomas M. File Jr,6 Alicia M. Fry,1 Stefan Gravenstein,7 Frederick G. Hayden,8 Scott A. Harper,9 Jon Mark Hirshon,10 Michael G. Ison,11 B. Lynn Johnston,12 Shandra L. Knight,13 Allison McGeer,14 Laura E. Riley,15 Cameron R. Wolfe,16 Paul E. Alexander,17,18 and Andrew T. Pavia19 1Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia; 2Division of General Pediatrics, Cohen Children’s Medical Center, New Hyde Park, New York; 3Division of Infectious Diseases, Rady Children’s Hospital, and 4University of California, San Diego; 5Department of Pediatrics, University of Washington, Seattle Children’s Hospital; 6Division of Infectious Diseases Summa Health, Northeast Ohio Medical University, Rootstown; 7Providence Veterans Affairs Medical Center and Center for Gerontology and Healthcare Research, Brown University, Providence, Rhode Island; 8Division of Infectious Diseases and International Health, University of Virginia Health System, Charlottesville; 9Office of Public Health Preparedness -

Part 4 May 2016 Sulfadoxine/Pyrimethamine 500Mg/25Mg, Tablets, (Guilin Pharmaceutical Co., Ltd), MA066
Artesunate 50mg Tablets + WHOPAR part 4 May 2016 Sulfadoxine/Pyrimethamine 500mg/25mg, Tablets, (Guilin Pharmaceutical Co., Ltd), MA066 SUMMARY OF PRODUCT CHARACTERISTICS 1 Artesunate 50mg Tablets + WHOPAR part 4 May 2016 Sulfadoxine/Pyrimethamine 500mg/25mg, Tablets, (Guilin Pharmaceutical Co., Ltd), MA066 1. NAME OF THE MEDICINAL PRODUCT ARTECOSPE ® 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each artesunate tablet contains 50 mg artesunate, each sulfadoxine/pyrimethamine tablet contains 500 mg sulfadoxine and 25 mg pyrimethamine. Each artesunate tablet contains 15 mg of sucrose. Each sulfadoxine/pyrimethamine tablet contains 20 mg of lactose. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM The artesunate 50 mg tablets are white round tablets debossed with “AS ” on one side and a score line on the other side. The sulfadoxine/pyrimethamine tablets (500/25 mg) are white, round, and debossed with “SP” on both sides, with a score line on one side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indication ARTECOSPE® (artesunate + sulfadoxine/pyrimethamine) is indicated for the treatment of uncomplicated cases of malaria due to Plasmodium falciparum strains which are susceptible to artesunate as well as to sulfadoxine /pyrimethamine. The most recent official guidelines on the appropriate use of antimalarial agents and local information on the prevalence of resistance to antimalarial drugs must be taken into consideration for deciding on the appropriateness of therapy with artesunate tablets and sulfadoxine/pyrimethamine tablets. Official guidance will normally include WHO (http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf?ua=1&ua=1) and public health authorities’ guidelines (see also sections 4.4 and 5.1). -

Manual for the Microscopical Diagnosis of Malaria in Man
NATIONAL INSTITUTE OP HEALTH Bulletin No. 180 MANUAL FOR THE MICROSCOPICAL DIAGNOSIS OF MALARIA IN MAN Federal Security Agency U. S. PUBLIC HEALTH SERVICE Washington, D. C. FEDERAL SECURITY AGENCY U. S. PUBLIC HEALTH SERVICE National Institute of Health Bulletin No. 180 MANUAL FOR THE MICROSCOPICAL DIAGNOSIS OF MALARIA IN MAN By AIMEE WILCOX, Assistant Technologist U. S. Public Health Service From the Division of Infectious Diseases National Institute of Health UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1942 For sale by the Superintendent ofDocuments, Washington, D. C. - - - - - - Price 30 cents ORGANIZATION OF THE NATIONAL INSTITUTE OF HEALTH Thomas Parran, Surgeon General, United States Public Health Service Dyer, R. E, Director, National Institute of Health Division of Biologics Control.—Chief, Senior Surgeon M. Y. Yeldee. Division of Chemistry.—Chief, Professor C. S. Hudson. Division of Chemotherapy.—Chief, Surgeon W. H. Sebrell, Jr. Division of Industrial Hygiene.—Chief, Medical Director J. G. Townsend. Division of Infectious Diseases. —Chief, Senior Surgeon Charles Armstrong. Division of Pathology.—Chief, Senior Surgeon R. D. Lillie. Division of Public Health Methods. —Chief, G. St.J. Perrott. Division of Zoology.-—Chief, Professor W. H. Wright. National Cancer Institute. —Chief, Pharmacologist Director Carl Voegtlin. FOREWORD This manual begins with a description of the morphology and life history of the parasites of the different species of malaria, a descrip- tion which is clear and thorough and should be useful to both the beginner in the subject and to one who may wish a concise review. The author uses throughout the terminology recommended by the Sub-Committee of the Health Organization of the League of Nations.