Update Tot 30-04-2020 1. Chloroquine and Hydroxychloroquine for The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Doxycycline and Hydroxychloroquine As Treatment for High-Risk COVID-19 Patients: Experience
medRxiv preprint doi: https://doi.org/10.1101/2020.05.18.20066902; this version posted May 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Doxycycline and Hydroxychloroquine as Treatment for High-Risk COVID-19 Patients: Experience from Case Series of 54 Patients in Long-Term Care Facilities Imtiaz Ahmad, MD, MPH, FCCP1 Mohammud Alam, MD2 Ryan Saadi, MD, MPH3,6 Saborny Mahmud4 Emily Saadi, BS5 1Allergy, Sleep & Lung Care, 21st Century Oncology, Fort Myers, FL 2Infectious Disease Specialist, Cordial Medical PC, Farmingdale, NY 3Center for Market Access and Medical Innovation, Warren, NJ 4 Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 5 Yale University, School of Public Health, New Haven, CT 6Quantaira Health, New York, NY Corresponding author : Imtiaz Ahmad, MD, Allergy, Sleep & Lung Care, 21st Century Oncology, Fort Myers, FL. email : [email protected] NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/2020.05.18.20066902; this version posted May 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Abstract: Importance: Patients in long-term care facilities (LTCF) are at a high-risk of contracting COVID-19 due to advanced age and multiple comorbidities. -
Neena Valecha1, Deepali Savargaonkar1, Bina Srivastava1, B
Valecha et al. Malar J (2016) 15:42 DOI 10.1186/s12936-016-1084-1 Malaria Journal RESEARCH Open Access Comparison of the safety and efficacy of fixed‑dose combination of arterolane maleate and piperaquine phosphate with chloroquine in acute, uncomplicated Plasmodium vivax malaria: a phase III, multicentric, open‑label study Neena Valecha1, Deepali Savargaonkar1, Bina Srivastava1, B. H. Krishnamoorthy Rao2, Santanu K. Tripathi3, Nithya Gogtay4, Sanjay Kumar Kochar5, Nalli Babu Vijaya Kumar6, Girish Chandra Rajadhyaksha7, Jitendra D. Lakhani8, Bhagirath B. Solanki9, Rajinder K. Jalali10, Sudershan Arora10, Arjun Roy10, Nilanjan Saha10, Sunil S. Iyer10, Pradeep Sharma10 and Anupkumar R. Anvikar1* Abstract Background: Chloroquine has been the treatment of choice for acute vivax malaria for more than 60 years. Malaria caused by Plasmodium vivax has recently shown resistance to chloroquine in some places. This study compared the efficacy and safety of fixed dose combination (FDC) of arterolane maleate and piperaquine phosphate (PQP) with chloroquine in the treatment of uncomplicated vivax malaria. Methods: Patients aged 13–65 years with confirmed mono-infection of P. vivax along with fever or fever in the previ- ous 48 h were included. The 317 eligible patients were randomly assigned to receive FDC of arterolane maleate and PQP (n 159) or chloroquine (n 158) for 3 days. Primaquine was given as an anti-relapse measure on day 3 and continued= for 14 consecutive days.= Primary efficacy analysis included assessment of the proportion of aparasitaemic and afebrile patients at 72 h. Safety endpoints were analysis of adverse events, vital signs, laboratory data, and abnor- malities on electrocardiograph. Patients participated in the study for at least 42 days. -

Online Supplement to Incremental Costs for Psoriasis and Psoriatic
Online supplement to Incremental Costs for Psoriasis and Psoriatic Arthritis in a Population-based Cohort in Southern Sweden: Is It All Psoriasis-attributable Morbidity? The Journal of Rheumatology, doi:10.3899/jrheum.150406 Table 1. ATC-codes used to define DMARDs and topical emollients Drug group ATC-code Generic name Biologic DMARDs L04AA24 Abatacept L04AB01 Etanercept L04AA21 Efalizumab* L04AB02 Infliximab** L04AB04 Adalimumab L04AB05 Certolizumabpegol L04AB06 Golimumab L04AC03 Anakinra L04AC05 Ustekinumab L04AC07 Tocilizumab L01XC02 Rituximab Non-biologic DMARDs A07EC01 Sulfasalazine D05BB02 Acitretin L04AA15 Leflunomid L04AD01 Ciklosporin L04AX01 Azathioprine L01BA01 Methotrexate L04AX03 M01CB01 Natriumaurotiomalat M01CB03 Auranofin P01BA01 Chloroquine P01 BA02 Hydroxychloroquine Topical emollients D05AA Tjäror D05AC01 Ditranol D05AX01 Fumarsyra D05AX02 Kalcipotriol D05AX52 Kalcipotriol + betametason D07AC01 Betametason D07CC01 Betametason + antibiotika D07AC17 Flutikason D07AC13 Mometason D07AB02 Hydrokortisonbutyrat D07AD01 Klobetasol D07AB01 Klobetason DMARD=Disease Modifying AntiRheumatic Drugs *Not on the market after 20090609 **Infliximab is given as infusion in hospitals and therefore not included in the cost component ”Drugs” in our presentation of resource use and associated costs. 1 Online supplement to Incremental Costs for Psoriasis and Psoriatic Arthritis in a Population-based Cohort in Southern Sweden: Is It All Psoriasis-attributable Morbidity? The Journal of Rheumatology, doi:10.3899/jrheum.150406 Table 2A. Mean annual -

Review Article Efforts Made to Eliminate Drug-Resistant Malaria and Its Challenges
Hindawi BioMed Research International Volume 2021, Article ID 5539544, 12 pages https://doi.org/10.1155/2021/5539544 Review Article Efforts Made to Eliminate Drug-Resistant Malaria and Its Challenges Wote Amelo 1,2,3 and Eyasu Makonnen 1,2 1Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, Addis Ababa University, Addis Ababa, Ethiopia 2Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), Addis Ababa University, Addis Ababa, Ethiopia 3Department of Pharmacology and Toxicology, School of Pharmacy, Jimma University, Jimma, Ethiopia Correspondence should be addressed to Wote Amelo; [email protected] Received 21 January 2021; Accepted 9 August 2021; Published 30 August 2021 Academic Editor: Jane Hanrahan Copyright © 2021 Wote Amelo and Eyasu Makonnen. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Since 2000, a good deal of progress has been made in malaria control. However, there is still an unacceptably high burden of the disease and numerous challenges limiting advancement towards its elimination and ultimate eradication. Among the challenges is the antimalarial drug resistance, which has been documented for almost all antimalarial drugs in current use. As a result, the malaria research community is working on the modification of existing treatments as well as the discovery and development of new drugs to counter the resistance challenges. To this effect, many products are in the pipeline and expected to be marketed soon. In addition to drug and vaccine development, mass drug administration (MDA) is under scientific scrutiny as an important strategy for effective utilization of the developed products. -

A Case of Hydroxychloroquine Induced Acute Generalized Exanthematous Pustulosis Confirmed by Accidental Oral Provocation
Ann Dermatol Vol. 22, No. 1, 2010 DOI: 10.5021/ad.2010.22.1.102 CASE REPORT A Case of Hydroxychloroquine Induced Acute Generalized Exanthematous Pustulosis Confirmed by Accidental Oral Provocation Jae-Jeong Park, M.D., Sook Jung Yun, M.D., Jee-Bum Lee, M.D., Seong-Jin Kim, M.D., Young Ho Won, M.D., Seung-Chul Lee, M.D. Department of Dermatology, Chonnam National University Medical School, Gwangju, Korea Acute generalized exanthematous pustulosis (AGEP) is a cytosis with an elevated neutrophil count. Spontaneous clinical reaction pattern that is principally drug induced and resolution usually occurs within 15 days without sequ- this is characterized by acute, nonfollicular sterile pustules elae. AGEP is induced mostly by drugs2, and especially on a background of edematous erythema. Hydroxychlo- antibiotics3. Annual incidence of AGEP is estimated to be roquine (HCQ) has been widely used to treat rheumatic and approximately 1 to 5 cases among one million persons2. dermatologic diseases and HCQ has been reported to be an Hydroxychloroquine (HCQ, oxyklorinTM, Myungmoon uncommon cause of AGEP. A 38-year-old woman with a Pharm. Co., Korea) has an antimalarial action and this 1-year history of dermatomyositis and polyarthralgia was drug is used for the treatment of rheumatic and der- treated with HCQ due to a lack of response to a previous matologic diseases due to its immunosuppressive and medication. Three weeks after starting HCQ therapy, the anti-inflammatory effects4. HCQ has been described as a pustular skin lesion developed and then this resolved after rare cause of AGEP in the Korean medical literature5. -
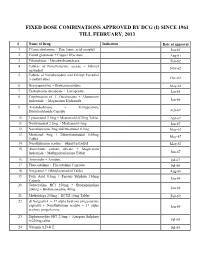
Fixed Dose Combinations Approved by Dcg (I) Since 1961 Till February, 2013
FIXED DOSE COMBINATIONS APPROVED BY DCG (I) SINCE 1961 TILL FEBRUARY, 2013 # Name of Drug Indication Date of approval 1. Cyanocobalamine + Zinc tannic acid complex Jan-61 2. Cobalt glutamate + Copper Glycinate Aug-61 3. Fibrinolysin + Desoxyribonuclease Feb-62 4. Tablets of Norethisterone acetate + Ethinyl Nov-62 oestradiol 5. Tablets of Norethynodrel and Ethinyl Estradiol 3-methyl ether Dec-62 6. Broxyquinoline + Brobenzoxalidine May-63 7. Testosterone decanoate + Isocaproate Jan-64 8. Combination of L Oxethazaine + Aluminium hydroxide + Magnesium Hydroxide Jun-66 9. Amylobarbitone + Trifluperazine Dihydrochloride Capsule Feb-67 10. Lynestronol 2.5mg + Mestranol 0.075mg Tablet Apr-67 11. Northynodrel 2.5mg + Mestranol 0.1mg Jun-67 12. Norethisterone 2mg and Mestranol 0.1mg May-67 13. Mestranol 4mg + Ethinyloestradial 0.05mg May-67 Tablet 14. Norethisterane acetate + ethinyl estradiol May-67 15. Aluminium sodium silicate + Magnesium hydroxide + Methypolysiloxane Tablet Jun-67 16. Ammoidin + Amidine Jul-67 17. Fluocortolene + Flucortolene Caproate Jul-68 18. Norgestrel + Ethinyloestradiol Tablet Aug-68 19. Folic Acid 0.5mg + Ferrous Sulphate 150mg Jan-69 Capsule 20. Tetracycline HCl 250mg + Broxyquinoline 200mg + Brobenzoxadine 40mg Jan-69 21. Methyldopa 250mg + HCTZ 15mg Tablet Feb-69 22. dl Norgestrel + 17 alpha hydroxy progesterone caproate + Norethisterone acetate + 17 alpha Jan-69 acetoxy progesterone 23. Diphenoxylate HCl 2.5mg + Atropine Sulphate 0.025mg tablet Jul-69 24. Vitamin A,D & E Jul-69 25. Lutin 0.1gm + Vit 0.1gm + Vit K1 2.5mg + Dicalcium Phosphate 0.1gm + Carlozochrome Jul-69 Salicylate 1mg tablet 26. Vit K 1 5mg + Calcium Lactolionate 100 m g+ Carlozocrome Salicylate 2.5mg + Phenol 0.5% Jul-69 + Lignocaine Hcl 1% injection 27. -

A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients
DOI: 10.14744/ejmo.2021.16263 EJMO 2021;5(1):63–70 Research Article A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients Abu Taiub Mohammed Mohiuddin Chowdhury,1 Mohammad Shahbaz,2 Md Rezaul Karim,3 Jahirul Islam, Guo Dan,1 Shuixiang He1 1Department of Gastroenterology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, P.R. China 2Chakoria Upazilla Health Complex, Cox’s Bazar, Bangladesh 3Biomedical Research Institute of Hubei University of Medicine, Shiyan, China 4Department of Epidemiology and Health Statistics, Xi’an Jiaotong University, Xi’an, Shaanxi, P.R. China Abstract Objectives: We investigated the outcomes of Ivermectin-Doxycycline vs. Hydroxychloroquine-Azithromycin combina- tion therapy in mild to moderate COVID19 patients. Methods: Patients were divided randomly into two groups: Ivermectin 200µgm/kg single dose + Doxycycline 100mg BID for ten days in group A, and Hydroxychloroquine 400mg for the first day, then 200mg BID for nine days + Azithro- mycin 500mg daily for five days in group B (Control group). RT-PCR for SARS-CoV-2 infection was repeated in all symp- tomatic patients on the second day onward without symptoms. Repeat PCR was done every two days onward if the result found positive. Time to the negative PCR and symptomatic recovery was measured for each group. Results: All subjects in Group A reached a negative PCR, at a mean of 8.93 days, and reached symptomatic recovery, at a mean of 5.93 days, with 55.10% symptom-free by the fifth day. In group B, 96.36% reached a negative PCR at a mean of 9.33 days and were symptoms-free at 6.99 days. -

Disease-Modifying Antirheumatic Drugs (Dmards) and Drug Interactions in Dentistry
European Review for Medical and Pharmacological Sciences 2021; 25: 2834-2842 Disease-Modifying Antirheumatic Drugs (DMARDs) and drug interactions in dentistry C. MUÑOZ-MARTÍNEZ, M. SEGURA-PUERTAS, G. GÓMEZ-MORENO Department of Medically Compromised Patients in Dentistry, School of Dentistry, University of Granada, Granada, Spain Abstract. – OBJECTIVE: Rheumatoid arthri- main symptoms. Its prevalence is five out of every tis is a chronic autoimmune disease. Treatment 1000 adults, affecting more women (aged 30-50 aims to reduce and improve its signs and symp- years) than men1,2. toms. Hence, Disease-Modifying Antirheumat- RA is the chronic inflammation of the synovial ic Drugs (DMARDs) are the treatment of choice. membrane and can advance to a point at which The objective of this study was to identify po- it destroys articular cartilage and juxta-articular tential interactions between DMARDs and the drugs most frequently prescribed in dentistry in bone. In addition, the patient suffers multiple order to avoid adverse reactions. organ disorders, swelling, pain, and joint rigidi- MATERIALS AND METHODS: This literature ty. After RA first appears, progressive articular review sets out to define possible adverse reac- destruction advances rapidly, causing deformed tions provoked by pharmacological interactions joints and unalterable physical abnormalities. between DMARDs and the drugs commonly pre- Appropriate diagnosis and treatment play in- scribed in dentistry. A search was conducted in PubMed by searching the names of drugs dispensable roles in dealing with this disease. used in dentistry, “drug interactions,” “rheuma- Ongoing research and developments in science toid arthritis,” and “dentistry”, “hydroxychlo- and technology have achieved major advances in roquine”, “leflunomide”, “methotrexate”, “sul- the remission of RA at early stages or in reducing fasalazine”, “adalimumab”, “anakinra”, “etaner- activity in established RA1. -

Prevalence of Hydroxychloroquine Retinopathy Using 2018 Royal College of Ophthalmologists Diagnostic Criteria
Eye (2021) 35:343–348 https://doi.org/10.1038/s41433-020-1038-2 ARTICLE Prevalence of hydroxychloroquine retinopathy using 2018 Royal College of Ophthalmologists diagnostic criteria 1 1 1 1 1 1 Elena Marshall ● Matt Robertson ● Satu Kam ● Alison Penwarden ● Paraskevi Riga ● Nigel Davies Received: 29 March 2020 / Revised: 1 June 2020 / Accepted: 9 June 2020 / Published online: 25 June 2020 © The Author(s), under exclusive licence to The Royal College of Ophthalmologists 2020 Abstract Introduction To measure the prevalence of hydroxychloroquine retinopathy in patients attending a hydroxychloroquine monitoring service using 2018 Royal College of Ophthalmologists diagnostic criteria. Methods A service evaluation audit of a hydroxychloroquine retinopathy monitoring service was undertaken. Results of Humphrey 10–2 field tests, spectral-domain optical coherence tomography and fundus autofluorescence were collected with data on dose, weight, duration of treatment, estimated glomerular filtration rate, and concurrent tamoxifen therapy. Visual field tests were assessed as reliable or unreliable, and classified as normal, hydroxychloroquine-like, poor test or related to other pathology. Cases of definite and possible retinopathy were identified using the 2018 RCOphth 1234567890();,: 1234567890();,: criteria. Results There were 1976 attendances over two years of 1597 patients. Seven hundred and twenty-eight patients had taken hydroxychloroquine for less than 5 years and 869 had taken hydroxychloroquine for 5 years or more. Fourteen patients were identified with definite hydroxychloroquine retinopathy (1.6%), and 41 patients with possible retinopathy (4.7%). Sixty- seven per cent of 861 visual fields were performed reliably, with 66.9% classified as normal, 24.9% as poor test, 5.2% hydroxychloroquine-like and 3.0% abnormal due to other pathology. -

PHARMACOLOGY of NEWER ANTIMALARIAL DRUGS: REVIEW ARTICLE Bhuvaneshwari1, Souri S
REVIEW ARTICLE PHARMACOLOGY OF NEWER ANTIMALARIAL DRUGS: REVIEW ARTICLE Bhuvaneshwari1, Souri S. Kondaveti2 HOW TO CITE THIS ARTICLE: Bhuvaneshwari, Souri S. Kondaveti. ‖Pharmacology of Newer Antimalarial Drugs: Review Article‖. Journal of Evidence based Medicine and Healthcare; Volume 2, Issue 4, January 26, 2015; Page: 431-439. ABSTRACT: Malaria is currently is a major health problem, which has been attributed to wide spread resistance of the anopheles mosquito to the economical insecticides and increasing prevalence of drug resistance to plasmodium falciparum. Newer drugs are needed as there is a continual threat of emergence of resistance to both artemisins and the partner medicines. Newer artemisinin compounds like Artemisone, Artemisnic acid, Sodium artelinate, Arteflene, Synthetic peroxides like arterolane which is a synthetic trioxolane cognener of artemisins, OZ439 a second generation synthetic peroxide are under studies. Newer artemisinin combinations include Arterolane(150mg) + Piperaquine (750mg), DHA (120mg) + Piperaquine(960mg) (1:8), Artesunate + Pyronardine (1:3), Artesunate + Chlorproguanil + Dapsone, Artemisinin (125mg) + Napthoquine (50mg) single dose and Artesunate + Ferroquine.Newer drugs under development including Transmission blocking compounds like Bulaquine, Etaquine, Tafenoquine, which are primaquine congeners, Spiroindalone, Trioxaquine DU 1302, Epoxamicin, Quinolone 3 Di aryl ether. Newer drugs targeting blood & liver stages which include Ferroquine, Albitiazolium – (SAR – 97276). Older drugs with new use in malaria like beta blockers, calcium channel blockers, protease inhibitors, Dihydroorotate dehydrogenase inhibitors, methotrexate, Sevuparin sodium, auranofin, are under preclinical studies which also target blood and liver stages. Antibiotics like Fosmidomycin and Azithromycin in combination with Artesunate, Chloroquine, Clindamycin are also undergoing trials for treatment of malaria. Vaccines - RTS, S– the most effective malarial vaccine tested to date. -

Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy a Report by the American Academy of Ophthalmology
Information Statement Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy A Report by the American Academy of Ophthalmology Michael F. Marmor, MD, Ronald E. Carr, MD, Michael Easterbrook, MD, Ayad A. Farjo, MD, William F. Mieler, MD, for the American Academy of Ophthalmology Introduction is not intended to be a review article, and only selected references are cited. Retinal toxicity from chloroquine and its analogue, hy- These suggestions may be varied according to the needs droxychloroquine, has been recognized for many years. The of individual patients, but provide a basic framework for the first reports concerned long-term usage of chloroquine for management of most patients. It cannot be emphasized too malaria, and later reports showed retinopathy in the treat- strongly that whatever screening regimen is followed, the ment of anti-inflammatory diseases. Chloroquine toxicity keys to early recognition of toxicity, and to the avoidance of remains a problem in many parts of the world, but is seen liability, are first informing the patient (and if possible the infrequently in the United States where the drug has largely primary care physician) of the risks and of the need for been replaced by hydroxychloroquine for the treatment of examinations, and second documenting these admonitions systemic lupus erythematosus, rheumatoid arthritis, and carefully in the record. These drugs are typically prescribed other inflammatory and dermatologic conditions. Retinal by internists, rheumatologists or dermatologists who may -

Screening for Hydroxychloroquine Maculopathy
Advances in Ophthalmology & Visual System Mini Review Open Access Screening for hydroxychloroquine maculopathy Abstract Volume 9 Issue 6 - 2019 Introduction: Hydroxychloroquine (HCQ) is an immunosuppressant widely used by Emad Selim,1 Soad Elsawy,2 Sanjeev Verma,1 rheumatologists in treatment of Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus 3 (SLE) and other conditions. Rehab Auf 1North Cumbria University Hospitals, UK Methods: Literature review on reasons why HCQ is commonly prescribed, its side effects 2Reumatology Department, Al Azhar University, Egypt and when it is practical to screen for them and what happen if those side effects develop. 3Howard University, Washington DC, USA Conclusion: Hydroxychloroquine is a disease modified medicine used in rheumatologic Correspondence: Emad Selim, department of Ophthalmology, diseases. Since retinal toxicity is normally irreversible and progressive even after cessation North Cumbria University Hospitals, UK, Tel +447429309328, of medicine, it essential to screen for early feature of retinal toxicity. Email Keywords: chloroquine, hydroxychloroquine, maculopathy, rheumatoid arthritis, Received: September 23, 2019 | Published: November 15, systemic lupus erythematosus, DMARMs 2019 Introduction being highest in the eye in the uveal tract and retinal pigment epithelium. Therefore retinal is the first part of the eye to suffer from Disease-modifying antirheumatic drugs (DMARDs) are a groups such toxicity. Such toxicity will be related to the dose of medicine of medicines used to alter the course of rheumatological conditions. used over a certain period of time reaching a certain cumulative dose Chloroquine was first introduced as an antimalarial drug in the Second and hence a certain level of concentration in the melanin pigments rich World War. In countries outside the United States, it is still primarily tissues.