Cranial Nerves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
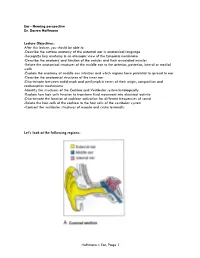
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Bedside Neuro-Otological Examination and Interpretation of Commonly
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2004.054478 on 24 November 2004. Downloaded from BEDSIDE NEURO-OTOLOGICAL EXAMINATION AND INTERPRETATION iv32 OF COMMONLY USED INVESTIGATIONS RDavies J Neurol Neurosurg Psychiatry 2004;75(Suppl IV):iv32–iv44. doi: 10.1136/jnnp.2004.054478 he assessment of the patient with a neuro-otological problem is not a complex task if approached in a logical manner. It is best addressed by taking a comprehensive history, by a Tphysical examination that is directed towards detecting abnormalities of eye movements and abnormalities of gait, and also towards identifying any associated otological or neurological problems. This examination needs to be mindful of the factors that can compromise the value of the signs elicited, and the range of investigative techniques available. The majority of patients that present with neuro-otological symptoms do not have a space occupying lesion and the over reliance on imaging techniques is likely to miss more common conditions, such as benign paroxysmal positional vertigo (BPPV), or the failure to compensate following an acute unilateral labyrinthine event. The role of the neuro-otologist is to identify the site of the lesion, gather information that may lead to an aetiological diagnosis, and from there, to formulate a management plan. c BACKGROUND Balance is maintained through the integration at the brainstem level of information from the vestibular end organs, and the visual and proprioceptive sensory modalities. This processing takes place in the vestibular nuclei, with modulating influences from higher centres including the cerebellum, the extrapyramidal system, the cerebral cortex, and the contiguous reticular formation (fig 1). -

Hearing Loss, Vertigo and Tinnitus
HEARING LOSS, VERTIGO AND TINNITUS Jonathan Lara, DO April 29, 2012 Hearing Loss Facts S Men are more likely to experience hearing loss than women. S Approximately 17 percent (36 million) of American adults report some degree of hearing loss. S About 2 to 3 out of every 1,000 children in the United States are born deaf or hard-of-hearing. S Nine out of every 10 children who are born deaf are born to parents who can hear. Hearing Loss Facts S The NIDCD estimates that approximately 15 percent (26 million) of Americans between the ages of 20 and 69 have high frequency hearing loss due to exposure to loud sounds or noise at work or in leisure activities. S Only 1 out of 5 people who could benefit from a hearing aid actually wears one. S Three out of 4 children experience ear infection (otitis media) by the time they are 3 years old. Hearing Loss Facts S There is a strong relationship between age and reported hearing loss: 18 percent of American adults 45-64 years old, 30 percent of adults 65-74 years old, and 47 percent of adults 75 years old or older have a hearing impairment. S Roughly 25 million Americans have experienced tinnitus. S Approximately 4,000 new cases of sudden deafness occur each year in the United States. Hearing Loss Facts S Approximately 615,000 individuals have been diagnosed with Ménière's disease in the United States. Another 45,500 are newly diagnosed each year. S One out of every 100,000 individuals per year develops an acoustic neurinoma (vestibular schwannoma). -

How Does Psychological Trauma Affect the Body and the Brain the Cortex the Limbic System
How Does Psychological Trauma Affect the Body and the Brain It would take many volumes to thoroughly discuss the brain in total. In this book I will stick to an overview discussion of the parts of the brain that are most relevant to the essential understanding of trauma: the cortex (the thinking center of the brain) and the Iimbic system (the emotional and survival center of the brain). The Cortex Among other functions, the cortex is the site of conscious thought and awareness. Maintaining attention to our external environment (what we see, hear, smell, etc.) as well as our internal environment (thoughts, body sensations, and emotions) requires activity in the cortex. Thinking, including the recall of facts, description of procedures, recognition of time, understanding, and so on, also takes place in the cortex. Though it varies from individual to individual, low levels of increased stress with the accompanying increase in adrenaline levels will actually improve awareness, clear thinking, and memory.1 That is why coffee is such a popular beverage at work and among university students: a jolt of caffeine makes our memory, observations, and thinking processes sharper. However, past a certain (individually determined) level, increased adrenaline will degrade, that is, have the opposite effect on, those same processes. A most recognizable example is seen on television quiz programs. More often than not, contestants eliminated by a wrong answer will assert that when watching the program at home, they never missed an answer. Why then were they stumped when on TV? Most likely, their stress levels rose beyond the helpful low-adrenaline kick and succumbed to overload that dampened their ability to access information that was easily available under calmer circumstances. -

Technische Universität München
TECHNISCHE UNIVERSITÄT MÜNCHEN Lehrstuhl für Entwicklungsgenetik Molecular mechanisms that govern the establishment of sensory-motor networks Rosa-Eva Hüttl Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzender: Univ.-Prof. Dr. E. Grill Prüfer der Dissertation: 1. Univ.-Prof. Dr. W. Wurst 2. Univ.-Prof. Dr. H. Luksch Die Dissertation wurde am 08.12.2011 bei der Technischen Universität München eingereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt am 27.02.2012 angenommen. Erklärung Hiermit erkläre ich an Eides statt, dass ich die der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Promotionsprüfung vorgelegte Arbeit mit dem Titel „Molecular mechanisms that govern the establishment of sensory-motor networks“ am Lehrstuhl für Entwicklungsgenetik unter der Anleitung und Betreuung durch Univ.-Prof. Dr. Wolfgang Wurst ohne sonstige Hilfe erstellt und bei der Abfassung nur die gemäß § 6 Abs. 5 angegebenen Hilfsmittel benutzt habe. Ich habe keine Organisation eingeschaltet, die gegen Entgelt Betreuerinnen und Betreuer für die Anfertigung von Dissertationen sucht, oder die mir obliegenden Pflichten hinsichtlich der Prüfungsleistung für mich ganz oder teilweise erledigt. Ich habe die Dissertation in dieser oder -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Phillips 2012 an Examination of the Efficacy of Sensory Integration in Occupational Therapy.Pdf
A Senior Honors Thesis February 2012 An Examination of the Efficacy of Sensory Integration in Occupational Therapy Shannon Phillips If an individual with sensory processing disorder undergoes occupational therapy treatment with the incorporation of sensory integration techniques, he or she will show measurable improvements in overall functioning, i.e., tactile sensitivity, taste/smell sensitivity, movement sensitivity, seeking sensation or under-responsiveness, and visual/auditory sensitivity. ACKNOWLEDGMENTS I would like to thank and acknowledge the many people who have helped make this Thesis of An Examination of the Efficacy of Sensory Integration in Occupational Therapy a success. First of all, to my advisor Mrs. Betty Marko, thank you for all the time spent with me in meetings, reading and revising my project, and giving me such wonderful guidance, advice, and insight. I very much appreciate all you have done and more. To my reader, Dr. Bob Humphries, thank you for your generous suggestions and for taking the time to help read and revise my project. To Dr. Laci Fiala Ades, thank you so much for all your help with my data consolidation. Without you as my statistical liaison, I would not have been able to convey my results in such an informative and intelligent manner. To Dr. Koop Berry and the rest of the Honors Committee, thank you for presenting me with this opportunity to come up with original research regarding my interest in occupational therapy. I know this experience can only help in my journey to graduate school, and it has left me with valuable lessons for life as well. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Sensory Processing Disorder with Strategies for Learning Behavior
Sensory Processing Disorder with Strategies for learning Behavior and Social Skills Description: Understanding and learning to recognize the sensory needs of the children who have spectrum processing disorder. Bonnie Vos, MS, O.T.R./L History of Diagnosis Autism criterion Autism not it’s better defined, # Subtype own diagnostic of diagnosis s are category increased rapidly DSM I DSM II DSM II DSM DSM IV DSM V 1952 1968 1980 III-R 1992 2013 1987 Autism not it’s Autism New subtypes are own diagnostic becomes its added, now 16 category own diagnostic behaviors are category characteristic (must have 6 to qualify) History of Diagnosis 1. New name (Autism Spectrum Disorder) 2. One diagnosis-no subcategories 3. Now 2 domains (social and repetitive) 4. Symptom list is consolidated 5. Severity rating added 6. Co-morbid diagnosis is now permitted 7. Age onset criterion removed 8. New diagnosis: Social (pragmatic) Communication disorder What is Autism • http://www.parents.com/health/autism/histo ry-of-autism/ Coding of the brain • One theory of how the brain codes is using a library metaphor. • Books=experiences • Subjects areas are representations of the main neuro- cognitive domains: • The subjects are divided into 3 sections: 1. Sensory: visual, auditory, ect. 2. Integrated skills: motor planning (praxis), language 3. Abilities: social and cognitive • Designed as a model for ideal development The Brain Library • Each new experience creates a book associated with the subject area that are active during the experience • Books are symbolic of whole or parts of experiences • The librarian in our brain – Analyzes – Organizes – Stores – Retrieves Brain Library • The earliest experiences or sensory inputs begin writing the books that will eventually fill the foundational sections of our brain Libraries – Sensory integration begins in-utero – Example: vestibular=14 weeks in-utero – Example: Auditory=20 weeks in-utero Brain Library 1. -

Nociceptor Sensory Neuron–Immune Interactions in Pain and Inflammation
Feature Review Nociceptor Sensory Neuron–Immune Interactions in Pain and Inflammation 1,2 2 Felipe A. Pinho-Ribeiro, Waldiceu A. Verri Jr., and 1, Isaac M. Chiu * Nociceptor sensory neurons protect organisms from danger by eliciting pain Trends and driving avoidance. Pain also accompanies many types of inflammation and A bidirectional crosstalk between noci- injury. It is increasingly clear that active crosstalk occurs between nociceptor ceptor sensory neurons and immune cells actively regulates pain and neurons and the immune system to regulate pain, host defense, and inflamma- inflammation. tory diseases. Immune cells at peripheral nerve terminals and within the spinal cord release mediators that modulate mechanical and thermal sensitivity. In Immune cells release lipids, cytokines, and growth factors that have a key role turn, nociceptor neurons release neuropeptides and neurotransmitters from in sensitizing nociceptor sensory neu- nerve terminals that regulate vascular, innate, and adaptive immune cell rons by acting in peripheral tissues and responses. Therefore, the dialog between nociceptor neurons and the immune the spinal cord to produce neuronal plasticity and chronic pain. system is a fundamental aspect of inflammation, both acute and chronic. A better understanding of these interactions could produce approaches to treat Nociceptor neurons release neuropep- chronic pain and inflammatory diseases. tides that drive changes in the vascu- lature, lymphatics, and polarization of innate and adaptive immune cell Neuronal Pathways of Pain Sensation function. Pain is one of four cardinal signs of inflammation defined by Celsus during the 1st century AD (De Nociceptor neurons modulate host Medicina). Nociceptors are a specialized subset of sensory neurons that mediate pain and defenses against bacterial and fungal densely innervate peripheral tissues, including the skin, joints, respiratory, and gastrointestinal pathogens, and, in some cases, neural fi tract. -

Positive Perilymph Fistula Test with Semicircular Canal Dehiscence from Cholesteatoma
PRACTICE | CLINICAL IMAGES Positive perilymph fistula test with semicircular canal dehiscence from cholesteatoma Ming-Chih Hsieh MD, Chen Chi Wu MD PhD, Shih-Hao Wang MD n Cite as: CMAJ 2019 January 28;191:E104. doi: 10.1503/cmaj.180799 54-year-old man presented to our outpatient department with left-side hearing loss and tinnitus that had progressed for several years. The patient had vertigo with nausea, whichA was aggravated on applying pressure over the left external ear canal and tragus. Physical examination showed left-side tym- panic membrane retraction with a whitish mass at the epitympa- num, suggestive of cholesteatoma. Gently compressing the left-ear tragus induced apparently left-beating horizontal nystagmus (see video, Appendix 1, available at www.cmaj.ca/lookup/suppl/ doi:10.1503/cmaj.180799/-/DC1), consistent with a positive peri- lymph fistula test. Pure tone audiometry showed mixed-type hear- ing loss of 104 dB in the patient’s left ear and sensorineural hearing loss of 62 dB in his right. High-resolution computed tomography (CT) scan of the patient’s temporal bone showed a soft-tissue mass in his left middle ear and mastoid cavity with left lateral semicircular canal erosion (Appendix 2, available at www.cmaj.ca/lookup/suppl/ Figure 1: Microscopic view of left lateral semicircular canal dehiscence with doi:10.1503/cmaj.180799/-/DC1). These findings were compatible erosion of bony and membranous sections (arrows) in a 54-year-old man with with cholesteatoma with lateral semicircular canal dehiscence. cholesteatoma. Note: *the malleus handle; +second genu of the facial nerve; During surgery, we noted that the osseous and membranous por- dotted lines define the tympanic segment of the facial nerve. -

Patient Information – Ear Surgery Instructions
The Oregon Clinic, Plaza ENT Division 5050 NE Hoyt #655, Portland, OR 97213 Phone: 5034882400 Fax: 5032310121 Patient Information – Ear Surgery Instructions Pre- and Post-operative Instructions for Ear Surgery (not including ear tubes) Before Surgery: Many ear surgeries involve manipulation of the eardrum (tympanic membrane), and some require the removal of bone to facilitate the treatment of your ear disease. As with any operation, infection, scarring, and blood clot formation (hematoma) are possible. The facial nerve is at risk for injury or temporary weakness during any ear surgery. Dizziness following surgery may be expected. Hearing loss or ringing in the ear (tinnitus) may be more pronounced. Taste disturbance is not uncommon in certain ear surgeries for a few weeks following surgery and, in a few instances, could be prolonged or permanent. An incision may be made behind your ear, on your earlobe, or behind the pointed cartilage in front of your ear (the tragus). These areas normally heal without problems or obvious scars. Hair around the ear may or may not be shaved. Flying is usually permitted one month after surgery. Swimming may be allowed six weeks after surgery, but check with your doctor first before resuming swimming or other water sports. If your work is not strenuous and depending upon the type of surgery you’ve had, you may return to work 3 to 4 days from the date of surgery. Generally, you will be seen about 2-3 weeks after surgery. This gives your eardrum time to heal before we see you back. Pre-operative Instructions: 1.