Product Recalls: a Remedy in Need of Repair
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcript (299.22
Internet of Things FTC Workshop November 19, 2013 Segment 4 Transcript >> WE'RE GOING TO GET STARTED. THIS IS PANEL THREE. THIS IS ON THE CONNECTED CARS. THIS IS PANEL THREE ON CONNECTED CARS. I'M KAREN JAGIELSKI AND I AM JOINED BY MY CO-MODERATOR. WIRE GOING TO INTRODUCE THE PANELISTS IN JUST A MINUTE. THIS IS A SHORT PANEL. WE ONLY HAVE AN HOUR, SO WE'RE GOING TO QUICKLY GET THROUGH INTRODUCTIONS AND THEN GET TO THE HEART OF THE SITUATION. SO WITH THAT, I'D ASK MY PANELISTS TO INTRODUCE THEMSELVES AND TELL US JUST A LITTLE BIT ABOUT YOURSELVES. >> HI, MY NAME IS TADAYOSHI KOHNO AND I'M A PROFESSOR AT THE UNIVERSITY OF WASHINGTON. MY AREA OF EXPERTISE IS COMPUTER SECURITY. ONE FOCUS WE LOOK AT IS SECURITY FOR CYBER SYSTEMS FOR MEDICAL DEVICES, HOME AUTOMATION, CHILDREN TOISES AND FOR PURPOSES OF TODAY TALKING ABOUT THE WORK WE'VE BEEN DOING FOR THE SECURITY AND PRIVACY OF THE MODERN AUTOMOBILE. >> I'M CHRIS WOLF, FOUNDER AND CO-CHAIR OF THE FUTURE PRIVACY FORUM. I LEAD THE PRIVACY PRACTICE AT HOGAN LEVELS AT THE FUTURE PRIVACY FORUM. WE'VE BEEN DOING A LOT OF WORK IN THE FIVE YEARS WE'VE BEEN AROUND ON THE INTERNET OF THING STARTING WITH OUR EFFORTS ON THE CODE OF CONDUCT ON THE SMART GRID, MORE RECENTLY DEALING WITH RETAIL LOCATION STANDARDS AND WE ALSO HAVE A CONNECTED PAR PROJECT THAT'S GOING ON AT FBF. TODAY WE PUBLISHED A PAPER CALLED AN UPDATED PRIVACY PARADIGM FOR THE INTERNET OF THINGS AND I GUESS I'LL TALK A LITTLE BIT ABOUT THAT DURING THE PANEL. -

Product Retrieval Procedures
PRODUCT RETRIEVAL PROCEDURES X-1 INDEX PAGE Overview 3 Analysis of FDA Recall Guidelines 4 A Product Retrieval Blueprint for Action 11 Food & Drug Regulations Title 21, Chapter 1 32 Subchapter A, Parts 7, 7.1 through 7.49 Method for Conducting Retrieval Effectiveness Checks 46 Published by Food and Drug Administration Example -- Corporate Retrieval Program 56 X-2 FOOD AND DRUG RECALL GUIDELINES OVERVIEW The regulations set forth in the Federal Register on June 16, 1978, established the following facts: 1. If an emergency of retrieval arises, it is the responsibility of a manufacturer or distributor to initiate voluntarily and carry out a retrieval of its product that is found to be in violation of the Food and Drug Act. 2. The retrieval must be initiated when the manufacturer discovers or is informed of the infraction. 3. The burden in carrying out a retrieval is totally that of a manufacturer or distributor. 4. Although a retrieval will be conducted by a manufacturer or distributor, it must be carried out to satisfaction of the FDA. To be able to conduct a product retrieval to the satisfaction of the FDA, the following preparation and conditions are essential: 1. An established contingency plan. 2. Assigned responsibility and authority to specific management personnel to carry out the contingency plan. 3. A thorough understanding of the regulation guidelines on retrieval. 4. Recognition of the urgency that FDA places on effectiveness, promptness and thoroughness. 5. Accurate documentation of ingredient and materials used. 6. Accurate documentation of distribution of products. 7. Accurate coding. The proof of effectiveness can only be learned through Trial Runs. -

Testimony for House EC on Self-Driving Cars 11-15-2016 Final
Statement of Laura MacCleery Vice President, Consumer Policy and Mobilization, Consumer Reports Before the U.S. House of Representatives Committee on Energy and Commerce Subcommittee on Commerce, Manufacturing, and Trade “Disrupter Series: Self-Driving Cars” Tuesday, November 15, 2016 Summary • Traffic deaths on U.S. roads rose to 35,092 last year and are estimated to have jumped another 10% in the first half of 2016. This is a public health crisis. We urgently need to find ways to prevent more traffic deaths and injuries and meaningfully counter this trend. • Crashworthiness improvements should continue or even be accelerated as an accompaniment to technological advances, and defects and recalls should be more aggressively overseen and pursued as warranted by the facts. • Automated driving systems—intended to yield self-driving cars—are advancing rapidly, and may be part of the solution. However, there is much more work that needs to be done to test and demonstrate safety benefits and protect consumers from novel risks. • This is particularly true regarding cars with semi-autonomous features, which if deployed irresponsibly can give consumers a dangerously false sense of security. • As the industry’s regulator, NHTSA can ensure that companies put consumers first by setting robust safety standards. NHTSA’s recent guidance rightly covers a wide range of important subjects, but it is light on specific steps companies must take to assure safety. • To protect the public and build trust in automated driving features, Congress should provide NHTSA the resources to independently and thoroughly assess the safety of automated systems and better understand how drivers interact with these new features. -

GLOBAL PRODUCT RECALL FOURTH EDITION Handbook
GLOBAL PRODUCT RECALL FOURTH EDITION Handbook Global Product Recall Handbook Fourth Edition Global Product Recall Handbook | Fourth Edition Foreword Baker McKenzie was founded in 1949. For almost seven decades, we have provided nuanced, sophisticated advice and leading-edge legal services to many of the world’s most dynamic and successful business organizations. With more than 7,000 internationally experienced lawyers in 47 countries, including 36 of the world’s largest economies, Baker McKenzie provides expertise in all of the substantive disciplines needed to formulate, develop and implement a global product recall. Our fluency in working across borders, issues and practices allows us to simplify legal complexity, foresee regulatory, legal, compliance, reputational, and commercial risks others may overlook and identify commercial opportunities that many miss. Taken together, this combination of deep practical experience and technical and substantive skills makes us advisers of choice to many of the world’s leading multinational corporations. Our clients want a new breed of lawyers with excellent technical skills and industry expertise who can look ahead to help them navigate a constantly changing product regulatory landscape. It means having lawyers who can anticipate what is coming next and provide practical legal resources that are helpful to the business at all levels. The Global Product Recall Handbook is one such resource, collecting, combining and synthesizing the advice of lawyers throughout Baker McKenzie focused on the consumer goods, pharmaceutical and medical devices, food and beverage, and motor vehicle industries. We are pleased also to make this edition of the Handbook available on a dedicated Dynamic Publisher site and accompanying mobile app. -

Nov. 2016 Edition OFFICE LOCATION
CONSUMER News Nov. 2016 Edition PROTECTION Dear Wisconsin Children’s Safety Advocates: In October 2016, the U.S. Consumer Product Safety Commission issued a total of 8 recalls relating to products affecting children. Attached is a summary of the releases identifying the product, the problem, and what should be done with the recalled product. We have found that not all of the recalls are picked up by the news media as they occur. This monthly summary will give you the opportunity to review all of the children’s product safety recalls for the past month. If you are interested in a complete text of the recall, double click on the hyperlink at the end of the recall description. This will direct you to the recall notice located on the CPSC website. Hallee Recalls Bed Canopies Due to Entanglement and Strangulation Hazards (17-701) FULLBEAUTY Brands Recalls Children’s Nightgowns Due to Violation of Federal Flammability Standard (17-702) Summer Infant Recalls Infant Bath Tubs Due to Risk of Impact Injury and Drowning (17-707) Mamas & Papas Recalls Armadillo Strollers Due to Fall Hazard (17-010) Roylco Recalls Educational Light Cubes Due to Fire Hazard WISCONSIN (17-012) DEPARTMENT OF Chimparoo Baby Carriers by L’echarpe Porte-bonheaur AGRICULTURE, Recalled Due to Fall Hazard (17-014) Target Recalls Halloween LED Gel Clings Due to Choking and TRADE AND Button Battery Ingestion Hazards (17-020) CONSUMER Fiddle Diddles Recalls Car Seat Strap Systems Due to PROTECTION Choking Hazard (17-705) If you would like to sign up for the Keep Your Kids Safe newsletter, please subscribe at https://public.govdelivery.com/accounts/WIDATCP/subscriber/new?topic_id OFFICE LOCATION =WIDATCP_161 or contact Bobbi Erb at (608) 224-4955 or [email protected]. -

Product Recalls Conceptualized As Social Dilemmas
PRODUCT RECALLS CONCEPTUALIZED AS SOCIAL DILEMMAS By SKYLER MASAJI KING A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON STATE UNIVERSITY Carson College of Business MAY 2016 © Copyright by SKYLER MASAJI KING, 2016 All Rights Reserved © Copyright by SKYLER MASAJI KING, 2016 All Rights Reserved To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of SKYLER MASAJI KING find it satisfactory and recommend that it be accepted. ___________________________________ Jeff Joireman, Ph.D., Chair ___________________________________ Andrew Perkins, Ph.D. ___________________________________ Joyce Ehrlinger, Ph.D. ii ACKNOWLEDGEMENT Throughout my time here at Washington State University, I have had the opportunity to meet exceptional scholars. First and foremost I would like to thank Dr. Jeff Joireman for his mentorship and guidance from day one. I could not have asked for a better mentor and friend throughout my time here. He has offered caring support throughout the program, allowing me to make and learn from my mistakes and never letting me take the easy way out through any process or project. Dr. Joireman, you have been a dedicated mentor and I sincerely hope to not only grow into a successful scholar like you are, but also grow into the type of person you are. I would also like to thank Drs. Andrew Perkins and Joyce Ehrlinger for the great resource they have been throughout the dissertation process. I feel very fortunate to have learned from them. Their academic pedigree is truly amazing. Additionally, their enthusiasm for research and helping students is second to none and I hope to emulate their knowledge and work ethic throughout my career in academia. -
The Negotiation Process
GW Law Faculty Publications & Other Works Faculty Scholarship 2003 The Negotiation Process Charles B. Craver George Washington University Law School, [email protected] Follow this and additional works at: https://scholarship.law.gwu.edu/faculty_publications Part of the Law Commons Recommended Citation Charles B. Craver, The Negotiation Process, 27 Am. J. Trial Advoc. 271 (2003). This Article is brought to you for free and open access by the Faculty Scholarship at Scholarly Commons. It has been accepted for inclusion in GW Law Faculty Publications & Other Works by an authorized administrator of Scholarly Commons. For more information, please contact [email protected]. THE NEGOTIATION PROCESS1 By Charles B. Craver2 I. INTRODUCTION Lawyers negotiate constantly. They negotiate on the telephone, in person, through the mail, and through fax and e-mail transmissions, They even negotiate when they do not realize they are negotiating. They negotiate with their own partners, associates, legal assistants, and secretaries; they negotiate with prospective clients and with current clients. They then negotiate on behalf of clients with outside parties as they try to resolve conflicts or structure business arrangements of various kinds. Most attorneys have not formally studied the negotiation process. Few have taken law school courses pertaining to this critical lawyering skill, and most have not read the leading books and articles discussing this topic. Although they regularly employ their bargaining skills, few actually understand the nuances of the bargaining process. When they prepare for bargaining encounters, they devote hours to the factual, legal, economic, and, where relevant, political issues. Most lawyers devote no more than ten to fifteen minutes on their actual negotiation strategy. -

Reasons to Buy: Contaminated Products & Recall Insurance
Casualty Reasons to Buy: Contaminated Products & Recall Insurance Almost any company involved in the food and beverage industry supply chain may be exposed to an accidental contamination and or recall exposure. Also, there is the possibility that a company’s particular brand may be the target of a malicious threat or extortion, either internally or from a third party. The potential financial impact of a product recall, as well as the lasting impact on brand reputation can be of serious concern to a company and its shareholders. AIG Contaminated Products & Recall Insurance not only protects against loss of gross profits and rehabilitation costs following either accidental or malicious contamination, but also provides the crisis management planning and loss prevention services of leading international crisis management specialists in food safety, brand & reputation impacts and extortion. Cover Cover is triggered by the recall of a product caused by: Accidental Contamination Any accidental or unintentional contamination, impairment or mislabeling which occurs during Cover Includes: or as a result of its production, preparation, manufacturing, packaging or distribution; provided • Recall costs that the use or consumption of such product has resulted in or would result in a manifestation of bodily injury, sickness, disease or death of any person within 120 days after consumption or use. • Business interruption (lost gross profit) Malicious Tampering • Rehabilitation costs Any actual, alleged or threatened, intentional, malicious and wrongful alteration or • Consultancy costs contamination of the Insured’s product so as to render it unfit or dangerous for use or consumption or to create such impression to the public, whether caused by employees or not. -

THE WESTFIELD LEADER the Leading and Most Widely Circulated Weekly Xeuspaper in Union County
THE WESTFIELD LEADER The Leading and Most Widely Circulated Weekly Xeuspaper In Union County I'ulillahed 28 Pages—15 Cents EIGHl WESTFIELD, KEW JERSEY, THURSDAY, DECEMBER 21. 1978 ICvery Thumday T ^e Drinking Arrests Triple in '78 $15.5 Million School In a joint effort this week teenage arrests related to school Christmas vacation, grounds, are used as an area Parents must know. too. to reduce the rising in- alcoholic consumption. To parents are urged to give where teenagers gather and there have been incidents of cidence of teenage date, for this year, the special attention to teenage have drinking and smoking students bringing alcoholic alcoholism, the Children. number has risen to 106 parties, remembering that • marijuanai parties. As a beverages to school. The Youth and Recreation arrests, and the year is not the use of alcohol by minors display of cooperation to law is very explicit that no Budget Anticipated Committee of P-T Council. over Included in these is not only illegal, but ex- combat teenage drinking, student, regardless uf age. working with the Westfield statistics are children ages tremely dangerous to their marijuana usage and may use alcoholic Police Department, 13 through 17. Most of these young bodies. Police vandalism, the Westfield beverages while attending Indications of a 1979-80 elementary advanced is not bad," he said. year. released the following in- youngsters were so in-statistics reveal, also an Board of Education gave school, or any school func- school budget in the neigh- learning centers for above The -

The Center for Auto Safety
CCEENNTTEERR FFOORR AAUUTTOO SSAAFFEETTYY 1825 Connecticut Avenue, NW Suite 330 Washington, DC 20009-1160 (202) 328-7700 Attachment A MISSION STATEMENT The Center for Auto Safety (CAS) is a nonprofit research and advocacy organization founded by Consumers Union and Ralph Nader in 1970 to provide consumers with a voice for auto safety and quality in Washington, D.C. and to assist owners of "lemon" vehicles to file complaints and obtain relief. Although CAS has a staff of less than a dozen people, its work is supported by approximately 20,000 members across the United States, and it is nationally recognized as a leader in the areas of automobile safety and consumer protection. CAS vigorously supports economically feasible motor vehicle safety policies that will reduce the risk of crash-related deaths and injuries. CAS serves as an important counterweight before federal policymakers to the automobile industry, whose positions on these safety issues are dictated by the desire to maximize profits for shareholders rather than to strike the proper balance between safety and other vehicle features. In fulfilling its mission, CAS is engaged in the following activities: Χ Researching defects in motor vehicles and monitoring defect investigations conducted by the National Highway Traffic Safety Administration (NHTSA) and other federal agencies; Χ Obtaining information on potential vehicle safety defects from consumers, alerting NHTSA to these problems, and requesting that NHTSA undertake investigations; Χ Responding with comments to agency rulemaking -
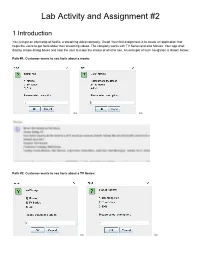
Lab Activity and Assignment #2
Lab Activity and Assignment #2 1 Introduction You just got an internship at Netfliz, a streaming video company. Great! Your first assignment is to create an application that helps the users to get facts about their streaming videos. The company works with TV Series and also Movies. Your app shall display simple dialog boxes and help the user to make the choice of what to see. An example of such navigation is shown below: Path #1: Customer wants to see facts about a movie: >> >> Path #2: Customer wants to see facts about a TV Series: >> >> >> >> Your app shall read the facts about a Movie or a TV Show from text files (in some other course you will learn how to retrieve this information from a database). They are provided at the end of this document. As part of your lab, you should be creating all the classes up to Section 3 (inclusive). As part of your lab you should be creating the main Netfliz App and making sure that your code does as shown in the figures above. The Assignment is due on March 8th. By doing this activity, you should be practicing the concept and application of the following Java OOP concepts Class Fields Class Methods Getter methods Setter methods encapsulation Lists String class Split methods Reading text Files Scanner class toString method Override superclass methods Scanner Class JOptionPane Super-class sub-class Inheritance polymorphism Class Object Class Private methods Public methods FOR loops WHILE Loops Aggregation Constructors Extending Super StringBuilder Variables IF statements User Input And much more.. -

Product Recalls, Imperfect Information, and Spillover Effects: Lessons from the Consumer Response to the 2007 Toy Recalls
NBER WORKING PAPER SERIES PRODUCT RECALLS, IMPERFECT INFORMATION, AND SPILLOVER EFFECTS: LESSONS FROM THE CONSUMER RESPONSE TO THE 2007 TOY RECALLS Seth M. Freedman Melissa Schettini Kearney Mara Lederman Working Paper 15183 http://www.nber.org/papers/w15183 NATIONAL BUREAU OF ECONOMIC RESEARCH 1050 Massachusetts Avenue Cambridge, MA 02138 July 2009 We gratefully acknowledge the helpful comments of Severin Borenstein, Jonathan Guryan, Judy Hellerstein, Ginger Jin, Arik Levinson, Soohyung Lee, Nuno Limao, and Abigail Wozniak as well as seminar participants at the University of Maryland, Rotman School of Management, the Energy Institute at U.C. Berkeley, Georgetown, UC-Davis, UC-Irvine, and University of Michigan. We thank Danny Kim at NPD for answering our questions about the toy sales data and Kevin Mak at the Rotman Finance Lab for his assistance with assembling the stock price data. Molly Reckson provided capable research assistance. Financial support from the AIC Institute for Corporate Citizenship at the Rotman School of Management is gratefully acknowledged. The views expressed herein are those of the author(s) and do not necessarily reflect the views of the National Bureau of Economic Research. NBER working papers are circulated for discussion and comment purposes. They have not been peer- reviewed or been subject to the review by the NBER Board of Directors that accompanies official NBER publications. © 2009 by Seth M. Freedman, Melissa Schettini Kearney, and Mara Lederman. All rights reserved. Short sections of text, not to exceed two paragraphs, may be quoted without explicit permission provided that full credit, including © notice, is given to the source. Product Recalls, Imperfect Information, and Spillover Effects: ¸˛Lessons from the Consumer Response to the 2007 Toy Recalls Seth M.