Chlorophyll in Australian Canola
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Catalogue Sheet
CATALOGUE SHEET - JOINT COPY PROJECT Uniting Church Archives of NSW & ACT Marriage Registers of the Presbyterian Church Society of Australian Genealogists – Sydney National Library of Australia - Canberra Mitchell Library – Sydney SAG Reel 346 0346 PLACE includes - OTHER PLACES START DATE END DATE Register References Albion Park 10-Apr-1954 5-Jul-1958 3 1 Albury 28-Aug-1946 11-Dec-1948 41 Albury 12-Jun-1948 19-Jul-1958 42 includes Corowa; Howlong Albury 19-Jul-1958 19-Dec-1959 43 Ardlethan 26-Feb-1913 20-Apr-1920 7 1 includes Narrandera; South Yalgogrin; Barellan; Beckom; Ariah Park; Bowlong; Kamarah; Colinrobbi; Grong Grong; Mirrool; Moombooldool; Ardlethan 21-Jun-1920 25-Apr-1942 7 2 includes Yalgogrin; Cootamundra; Wallaroobie; Barellan; Cowabbie; Beckom; Ardlethan 17-Dec-1947 17-Dec-1958 7 3 includes Barellan; Temora; Narrandera; Ariah Park; Weethalie; Mirrol; Arncliffe 9-Apr-1927 12-Nov-1930 91 Arncliffe 29-Jan-1931 26-Feb-1936 92 Arncliffe 8-Apr-1936 22-Oct-1938 93 Arncliffe 15-Apr-1939 9-May-1959 94 Auburn 18-Apr-1927 27-Apr-1929 13 1 Auburn [filmed out of date order] 7-Mar-1925 16-Apr-1927 38 4 The original records are held by Uniting Church Archives of NSW/ACT at North Parramatta and permission was granted for their microfilming to be undertaken by W & F Pascoe Pty Ltd in 2009. This microfilm is supplied for information and research purposes only. No reproduction in any form other than the copying of individual frames for research purposes is permitted without the assent of the Council of the Society of Australian Genealogists ‘Richmond Villa’ 120 Kent Street, Sydney, NSW 2000. -

NSW Trainlink Regional Train and Coach Services Connect More Than 365 Destinations in NSW, ACT, Victoria and Queensland
Go directly to the timetable Dubbo Tomingley Peak Hill Alectown Central West Euabalong West Condobolin Parkes Orange Town Forbes Euabalong Bathurst Cudal Central Tablelands Lake Cargelligo Canowindra Sydney (Central) Tullibigeal Campbelltown Ungarie Wollongong Cowra Mittagong Lower West Grenfell Dapto West Wyalong Bowral BurrawangRobertson Koorawatha Albion Park Wyalong Moss Vale Bendick Murrell Barmedman Southern Tablelands Illawarra Bundanoon Young Exeter Goulburn Harden Yass Junction Gunning Griffith Yenda Binya BarellanArdlethanBeckomAriah Park Temora Stockinbingal Wallendbeen Leeton Town Cootamundra Galong Sunraysia Yanco BinalongBowning Yass Town ACT Tarago Muttama Harden Town TASMAN SEA Whitton BurongaEuston BalranaldHay Carrathool Darlington Leeton NarranderaGrong GrongMatong Ganmain Coolamon Junee Coolac Murrumbateman turnoff Point Canberra Queanbeyan Gundagai Bungendore Jervis Bay Mildura Canberra Civic Tumut Queanbeyan Bus Interchange NEW SOUTH WALES Tumblong Adelong Robinvale Jerilderie Urana Lockhart Wagga Wondalga Canberra John James Hospital Wagga Batlow VICTORIA Deniliquin Blighty Finley Berrigan Riverina Canberra Hospital The Rock Laurel Hill Batemans Bay NEW SOUTH WALES Michelago Mathoura Tocumwal Henty Tumbarumba MulwalaCorowa Howlong Culcairn Snowy Mountains South Coast Moama Barooga Bredbo Albury Echuca South West Slopes Cooma Wangaratta Berridale Cobram Nimmitabel Bemboka Yarrawonga Benalla Jindabyne Bega Dalgety Wolumla Merimbula VICTORIA Bibbenluke Pambula Seymour Bombala Eden Twofold Bay Broadmeadows Melbourne (Southern Cross) Port Phillip Bay BASS STRAIT Effective from 25 October 2020 Copyright © 2020 Transport for NSW Your Regional train and coach timetable NSW TrainLink Regional train and coach services connect more than 365 destinations in NSW, ACT, Victoria and Queensland. How to use this timetable This timetable provides a snapshot of service information in 24-hour time (e.g. 5am = 05:00, 5pm = 17:00). Information contained in this timetable is subject to change without notice. -

Located Just Two Hours from Canberra in — Walk Sip — R South-West NSW, the Riverina Region Is the Place — Un It — a B to Simply Go with the Flow
WEEKENDER Located just two hours from Canberra in — Walk Sip — R south-west NSW, The Riverina region is the place — un it — a B to simply go with the flow. W re a — t t h s e a — k C ic S P — e — W — e ast ait — el ip C S e — p — ip F S i — — S W r H a ea — — lk H e h — — h a c R ee c t un S r — e — t a Breath a — B B F — — e e w w l e r e — r B B P i — — c p k p — o to t S C S — r e u — riv n Taste — D c e h v — ri T D as — te te — as Dr — T ive — Stop — See Just as the waters of the majestic Murrumbidgee award-winning wineries, breweries and other River wind through welcoming country towns and exceptional foodie experiences. spectacular natural landscapes, let yourself be carried From tasty treats to breath-taking scenery, heart- away by our rich history, generous hospitality and thumping adventure or heavenly hideaways – whatever delicious local produce. your reason for visiting, you’ll easily drift from one The Riverina produces some of Australia’s best unique encounter to the next. food and drink, so you won’t need to go far to find visitnsw.com/the-riverina | #visitriverina The Kidman Way 2020 Bushfire Affected Newell Highway These businesses were affected by the WEST bushfires of early 2020. Please contact WEETHALLE WYALONG them directly to confirm opening hours. THE RIVERINA BARELLAN POONCARIE GRIFFITH CARRATHOOL Ariah Park WHITTON Coolamon Cheese, Coolamon HAY TEMORA Burley FOOD, CAFES AND RESTAURANTS BALRANALD LEETON Canola Trail Griin Way Hume & Hovell Nest Cafe Tumbarumba Murrumbidgee DARLINGTON COOTAMUNDRA River POINT Walking -

"United by One Vision"
"United by One Vision" The History of Coolamon Shire CONTENTS 1) IN THE BEGINNING…………… “FROM SMALL BEGINNINGS COME GREAT THINGS” 2) THEY SERVED OUR TOWN “THERE IS NOTHING MORE DIFFICULT TO TAKE IN HAND, MORE PERILOUS TO CONDUCT OR MORE UNCERTAIN IN ITS SUCCESS THAN TO TAKE THE LEAD IN THE INTRODUCTION OF A NEW ORDER OF THINGS” 3) HISTORY OF INDUSTRIES “AND THE WHEELS WENT ROUND” 4) COOLAMON SHIRE COUNCIL “COMING TOGETHER IS A BEGINNING; KEEPING TOGETHER IS PROGRESS; WORKING TOGETHER IS SUCCESS” 5) HISTORY OF EDUCATION IN THE DISTRICT “WE ARE TO LEARN WHILE WE LIVE” 6) HISTORY OF RELIGIOUS ACTIVITIES “OUR FATHER WHO ART IN HEAVEN……..” IN THE BEGINNING ……… “FROM SMALL BEGINNINGS COME GREAT THINGS” COOLAMON - ORIGIN OF NAME It was originally proposed to call Coolamon "Kindra", after the run and parish name, but the Pastoral Authorities were of the opinion that his name, if adopted, would possibly cause confusion with Kiandra. A conference between the District Surveyor and the Railway Traffic Branch led to Coolamon being suggested and agreed to by all parties. "Coolamon" is an aboriginal name meaning "dish or vessel for holding food or water". A plan showing the northern boundary of Coolemon (Coolamon) Holes Run in 1870 shows a cluster of numerous water holes which he referred to as Coolamon Holes. This was the native name given to the holes and the origin of the name as applied today. The name being finalised and the extent of the village and suburban boundaries fixed, the village of Coolamon was gazetted on 3rd October, 1881. -
Find Your Local Brigade
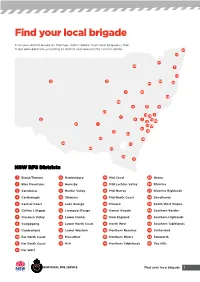
Find your local brigade Find your district based on the map and list below. Each local brigade is then listed alphabetically according to district and relevant fire control centre. 10 33 34 29 7 27 12 31 30 44 20 4 18 24 35 8 15 19 25 13 5 3 45 21 6 2 14 9 32 23 1 22 43 41 39 16 42 36 38 26 17 40 37 28 11 NSW RFS Districts 1 Bland/Temora 13 Hawkesbury 24 Mid Coast 35 Orana 2 Blue Mountains 14 Hornsby 25 Mid Lachlan Valley 36 Riverina 3 Canobolas 15 Hunter Valley 26 Mid Murray 37 Riverina Highlands 4 Castlereagh 16 Illawarra 27 Mid North Coast 38 Shoalhaven 5 Central Coast 17 Lake George 28 Monaro 39 South West Slopes 6 Chifley Lithgow 18 Liverpool Range 29 Namoi Gwydir 40 Southern Border 7 Clarence Valley 19 Lower Hunter 30 New England 41 Southern Highlands 8 Cudgegong 20 Lower North Coast 31 North West 42 Southern Tablelands 9 Cumberland 21 Lower Western 32 Northern Beaches 43 Sutherland 10 Far North Coast 22 Macarthur 33 Northern Rivers 44 Tamworth 11 Far South Coast 23 MIA 34 Northern Tablelands 45 The Hills 12 Far West Find your local brigade 1 Find your local brigade 1 Bland/Temora Springdale Kings Plains – Blayney Tara – Bectric Lyndhurst – Blayney Bland FCC Thanowring Mandurama Alleena Millthorpe Back Creek – Bland 2 Blue Mountains Neville Barmedman Blue Mountains FCC Newbridge Bland Creek Bell Panuara – Burnt Yards Blow Clear – Wamboyne Blackheath / Mt Victoria Tallwood Calleen – Girral Blaxland Cabonne FCD Clear Ridge Blue Mtns Group Support Baldry Gubbata Bullaburra Bocobra Kikiora-Anona Faulconbridge Boomey Kildary Glenbrook -

Country Train Notice 0292-2020 Country Regional Network
Country Train Notice 0292-2020 Country Regional Network Author: Beth Jarrett Issue Date: 23/10/2020 LTP Identification Number: 2286 Associated Publications: N/A Timetable for Heritage Operated Train The Rail Motor Society Paterson – Griffith Travelling under Manual Block Working Conditions as described in the TOC Manual Friday 30 October 2020 Consist: CPH 1, CPH 3 , CPH 7 [41m, 75T] 7R01 on Fri 30/10/2020 will run as tabled by ARTC to pass CRN/ARTC Boundary Stockinbingal 1422, Stockinbingal AWB Siding 1427, Combaning 1442, arrive Temora Sub Terminal 1455 depart 1500, arrive Temora 1505 depart 1530, pass Temora West (BFB) 1534, Pucawan 1551, Quandary 1554, Ariah Park Silo 1602, arrive Ariah Park 1605 depart 1750, pass Mirrool 1800, Beckom 1811, Ardlethan 1818, Ardlethan Silo 1819, Kamarah 1829, Moombooldool 1837, Barellan Silo 1845, Barellan 1845, Garoolgan 1855, Binya 1903, Yenda 1915, arrive Griffith 1930 – forms 7R02. Saturday 31 October 2020 Consist: CPH 1, CPH 3 , CPH 7 [41m, 75T] 7R02 on Sat 31/10/2020 will depart Griffith 1000, pass Yenda 1016, Binya 1028, Garoolgan 1036, Barellan 1047, Barellan Silo 1047, Moombooldool 1055, Kamarah 1104, Ardlethan Silo 1113, Ardlethan 1115, Beckom 1122, Mirrool 1133, arrive Ariah Park 1145 depart 1205, pass Ariah Park Silo 1207, Quandary 1215, Pucawan 1219, Temora West (BFB) 1236, Temora 1238, arrive Temora 1239 – forms 7R03. 7R03 on Sat 31/10/2020 will depart Temora 1250, pass Temora West (BFB) 1254, Pucawan 1311, Quandary 1314, Ariah Park Silo 1321, arrive Ariah Park 1325 depart 1530, pass Mirrool 1540, Beckom 1551, Ardlethan 1558, Ardlethan Silo 1559, Kamarah 1609, Moombooldool 1617, Barellan Silo 1625, Barellan 1625, Garoolgan 1635, Binya 1643, Yenda 1655, arrive Griffith 1710 – forms 7R04. -

Bland Temora Zone Bush Fire Management Committee Bush Fire Risk Management Plan 2020
Bland Temora Zone Bush Fire Management Committee Bush Fire Risk Management Plan 2020 Table of Contents Glossary 7 Chapter 1. Introduction 9 1.1 Background 9 1.2 Aim and Objectives 9 1.3 Description of the Bland Temora BFMC Area 10 1.3.1 Location and land tenure 10 1.3.2 Climate and bush fire season 10 1.3.3 Population and demographic information 10 1.3.4 History of bush fire frequency and ignition cause 11 Chapter 2. Identifying and Assessing the Bush Fire Risk 12 2.1 Process 12 2.2 Communication and Consultation 12 2.3 Identifying the Bush Fire Risk 13 2.3.1 Assets 13 2.3.2 Assessing the Bush Fire Risk - Consequence 14 2.3.3 Assessing the Bush Fire Risk - Likelihood 15 2.3.4 Identifying the level of risk 15 2.3.5 Evaluating the Bush Fire Risk 15 2.3.6 Prioritising Treatments 15 2.3.7 Risk Acceptability 16 Chapter 3. Treating the Risk 17 3.1 Bush Fire Management Zones 17 3.2 BFMC Wide Treatments 19 3.3 Asset Specific Treatments 20 3.4 Fire Thresholds 21 3.5 Annual Works Programs 22 3.6 Implementation 23 Chapter 4. Performance Monitoring and Reviewing 24 4.1 Review 24 4.2 Monitoring 24 4.3 Reporting 24 4.4 Performance Measurements 24 Appendix 1 Community Participation Strategy 25 Appendix 2 Asset Register 25 Appendix 3 Treatment Register 25 Appendix 4 Maps 25 Page 2 of 34 List of Tables Table 1.1 Land tenure 10 Table 3.1 Bush Fire Management Zones: Purpose, objectives and characteristics 18 Table 3.2 Asset specific treatments used in the (insert name here) BFMC area 21 Table 3.3 Fire Thresholds for Vegetation Categories 22 List of Figures Figure 2.1 Overview of the risk assessment process ................................................................................... -

2021-2024 Ariah Park Central School
Strategic Improvement Plan 2021-2024 Ariah Park Central School 1055 Page 1 of 8 Ariah Park Central School (1055) -2021-2024 Printed on: 1 April, 2021 School vision and context School vision statement School context We have an active culture of continuous improvement in our teaching, learning and Ariah Park Central School is located on the lands of the Wiradjuri people in the Riverina engagement with community at Ariah Park Central School. region of New South Wales. It is a rural K-12 school of 140 students who are drawn from the village of Ariah Park (population 493), nearby towns and the surrounding farmlands. The Central to the improvement in student performance will be our highly capable staff and latest census data show the main areas of employment are agricultural industries.. Trend students. Staff will exhibit high expectations in all that they do, with contextualized, focused data shows that enrolments in the school have been slowly increasing over the last decade. and evidence based professional learning as the foundation of our improvement. This will Currently 3% of students identify as Aboriginal and 1% of students identify as EAL/D. result in all school staff being dedicated to improving student outcomes. Our students will be independent, life long learners who are highly educated and well rounded people. Ariah Park Central School is part of the Riverina Access Partnership (RAP), one of 5 access programs that run in NSW. RAP provides a shared curriculum for senior secondary students The community will actively engage with our school to actively partner in their children's across six central schools that deliver Yr 11 and 12 studies across all six participating education. -

Open Day Last Friday Was a Great Success As Ariah Park Central School Celebrated Education Week. the Cappuccinos and Cake for Mo
2017 Term 3 August th 4A 7 Week The Newsletter of Monday, Ariah Park Central School Education Week Open Day Science Show Friday, 11/8/17 – D Worland, C Heward Open Day last Friday was a great success Monday, 14/8/17 – A Fairman, S Furphy as Ariah Park Central School celebrated Education Week. The cappuccinos and cake for morning tea received plenty of support, as did the Science Show and Dog P & C Meeting Training. There were some very colourful Our monthly meeting will be held canvases created for the different tomorrow evening, 8th August 2017, in seasons, which we will hope to see hung the school library, commencing at in the school, and the S.T.E.M. centre 7.30pm. We look forward to welcoming continued to be a hit with the motorised you. vehicles and coding lessons on display. Most popular of all, of course, was the Fly-In Ninja Warrior challenge in the school hall! The Temora Fly-in is Saturday, 19th August Thank you to all those staff, parents and and we are looking for volunteers to help students who contributed to the success man the canteen. We receive $500 to the of the day. P&C for doing this, which enables us to do This week continues to be busy. many things for your children. Please put Tomorrow evening is the monthly P&C your hand up for a shift. Contact Joel on Meeting and Friday will be the PSSA 0429 617 525. Athletics Carnival held here at Ariah Park, which we thank Ms Horne and our SASS staff for working hard to organise. -

Local History Books
Local History Library Our Search Room contains a small number of reference books, the majority of which are histories of community groups, schools, sporting groups, clubs, religious agencies and other topics that relate to our local area. Place Title Adelong Early Adelong – And Its Gold (W. Roy Ritchie) Historic Buildings of Adelong History and Happenings - St. Paul’s Anglican Church, Adelong – Sesquicentenary 1855 to 2005 (Parish Council) Albury The Faces and the Streets, Albury Wodonga 1955-2000, (Karen Donnelly) Ardlethan Poppet Heads and Wheatfields – A History of Ardlethan and District, South- West N.S.W. (Roy H. Taylor and Aub Griffiths) Ariah Park Ariah Park, Mirrool Football Club, 50 Years 1953-2003, (Shirley Bell) Mandamah West (Elizabeth Allen) Wowsers, Bowsers and Peppercorn Trees, (Nigel Judd) Australia A Checklist of Biographies of Australian Businessmen (La Trobe University) A Family Heritage (H.E. Fiveash) Australia’s Great River – The Murray Valley Past and present (R. M. Younger) Australian Universities, Colleges and Schools, Registry of Badges, Colours and Mottos, (Anthony Cree) Bendigo to Bowral – The Journey of a Lifetime (Joseph Lonsdale) Bicentennial, An Australian Mosaic and 1788 Diary, (Harry Gordon) Codswallop – Short Stories from the Upper Murray (Bill Robbins and Graham Jackson) Eleanor Rathbone and the Refugees (Susan Cohen) Exploration and Settlement in Australia, (James Gormly) Describing Archives in context: A guide to Australian Practice (The Australian society of Archivists committee on descriptive standards) Heritage Farming in Australia, The Sloane Family Changes and Challenges 1860-1945, (Alex and Anne Sloane) Into History – The Australian Historical Directory (Compiled by Ralph and Amy Reid) Into History – A Guide to Historical, Genealogical, Family History and Heritage Societies, Groups and Organisations in Australia. -

25Th APRIL 2016
25th APRIL 2016 Commemorative booklet proudly presented by Michael McCormack MP Federal Member for Riverina Assistant Minister for Defence WAR-TIME TOTS: A World War I postcard of an Australian patriotic scene showing two young girls dressed as nurses carrying a stretcher. WAR TOUGH ON CHILDREN OUR poignant cover of this year’s commemorative the south to the Bland and the South West Slopes in the ANZAC Day Riverina booklet features a caring nurse north, the Riverina has always contributed mightily in lovingly tending to a brave little trooper, wounded in battle. the nation’s darkest days. PITCHING IN: When it was war-time a woman’s work Role-playing the heroic deeds being done by the grown- War cost the Riverina heavily yet has never dented the was never done for if they weren’t nursing at the front, local ups at the front was one way children of The Great resolve of the region’s people to serve and to play their ladies were keeping the home fires burning and busy fund War era – indeed, littlies during any time of military part – at home or abroad. raising to help those fighting the good fight for God, King and conflict – were able to cope with the horrors of what was And now – as we mark a century since that terrible year CONTENTS Country. happening half a world away. of 1916 in which so many of our volunteer Diggers fell INSIDE: Playing dress-ups with siblings and friends was harmless at the killing fields of Bullecourt, Fromelles, Messines, and popular fun. -

Junee Roadside Vegetation Survey and Management Guidelines
CONTENTS FOREWORD........................................................................................................ 2 ACKNOWLEDGEMENTS .............................................................................................. 3 INTRODUCTION 1.1 The importance of roadside vegetation.................................................. 5 1.2 The Junee Roadside Vegetation Survey................................................ 6 1.3 Responsibility for road reserve management......................................... 7 1.4 Junee Shire – an overview..................................................................... 8 1.5 Survey Methodology .............................................................................. 8 SURVEY RESULTS 2.1 Roadside vegetation in Junee Shire .................................................... 12 2.2 Species Recorded................................................................................ 12 2.3 Native Plants........................................................................................ 14 2.4 Introduced Plants ................................................................................. 18 MANAGEMENT OF ROADSIDE VEGETATION 3.1 Management according to conservation status.................................... 21 3.2 Recommendations to Council .............................................................. 22 REFERENCES ...............................................................................................................25 APPENDICES A Junee Shire roadside vegetation data .......................................................26