Physico-Chemical Characterization And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Contributors to the West Bengal State Emergency Relief Fund
List of Contributors to the West Bengal State Emergency Relief Fund Amount of Affliating S/N Names of the Colleges Names of the Principal Contribution Univ (Rs) 1 Bardwan Balagarh Bijoy Krishna mahavidyalaya,(1985) Dr. Pratap Banerjee 64100.00 2 Bardwan Birbhum Mahavidyalaya,(1979) Dr. Parthasarathi Mukhopadhya 5000.00 3 Bardwan Chandidas Mahavidyalaya,(1972) Dr.Sk. Ataur Rahaman 39001.00 4 Bardwan Hooghly Women's College (1919)(W) Dr. Sima Banerjee 4000.00 5 Bardwan Jamalpur Mahavidyalaya Dr. Kartik Chandra Mukhopadhya 5237.00 6 Bankura Jamini Roy College (1986) Dr.Pradip Kumar Banerjee 260000.00 7 Bardwan Katwa College.(1948) (Day+ Evening) Dr. Nirmalendu Sarkar 4000.00 8 Bardwan Krishna Chandra College,(1897) Dr. Goutam Chatterjee 54500.00 9 Bardwan Mankar College, (1987) Dr.Sukanta Bhattacharyya 58500.00 10 Bardwan Rabindra Mahavidyalaya (1971) Dr.Prasanta Bhattacharya 50000.00 11 Bankura Saldiha College(1966) Dr. Shaikh Sirajuddin 5000.00 12 Bardwan Sambhu Nath College,(1963) Dr.Nisith Nath Chakravorty 111000.00 13 Bardwan Surya Sen Mahavidyalaya Dr. Md. Inamur Rahaman* 208000.00 14 Bardwan Syamsundar College,(1948) (W) Dr.Gouri Sankar Bandyopadhyay 6000.00 15 Bardwan Vivekananda Mahavidyalaya, Burdwan Dr.Sibaprasad Rudra 139100.00 16 CU Ananda Mohan College,(1961) Dr.Pradip Kumar Maity 6000.00 17 CU Asutosh College,(1916) Dr.Dipak Kumar Kar 1000000.00 18 CU Bagnan College (1958) Dr.Badal Kumar Maity 116287.00 19 CU Bhangar Mahavidyalaya,(1997) Dr.Virvikram Roy 5050.00 20 CU Bidhan Chandra College (1957) Dr.Sulekha Bose* 200000.00 -

University of North Bengal
UNIVERSITY OF NORTH BENGAL District: DARJEELING Sl. No. Name of the College & Address Phone No. Email-id 1. Bijonbari Degree College [1995] 0354-260201 Bijonbari- 734201 2. Cluny Women’s College [1998] Fax: 03552 257924 [email protected] Kalimpong -734301 3. Darjeeling Govt. College [1948] 0354-2254078 Lebong Court Road - 734101 Kalimpong College [1962] 4. Rishi Bankimchandra Park 03552-255231/255486 Kalimpong -734301 Kalipada Ghosh Tarai Mahavidyalaya 5. 0353 2551118 (Pl.) [email protected] [1988] Fax: 0353 2551662 Bagdogra-734422 Kurseong College [1967] 6. 0354 2344243 Down Hill Road, P.O. Kurseong – 734 Fax: 0354 2345667 203 Mirik College [2000] 0354 2243746 7. Old Dakbungalow, Thana Line Dr. Ananda Chhetri P.O. Mirik 734 214 9434329585 Salesian College [1933] 8. (self-financed) 0354-2222147 Sonada- 734219 Siliguri College [1950] 0353-2436590 9. [email protected] Collegepara, Siliguri- 734001 /2533191/2436590 10. Siliguri College of Commerce [1962] Siliguri-734401 0353-2432594/2436817 Siliguri Mahila Mahavidyalaya [1981] 11. 1 Dabgram Colony, Rabindra Sarani 0353-2596442 Siliguri- 734406 12. Sonada College [1985] 0354-2466316 Sonada- 734219 Southfield College [1961] 13. (Formerly Loreto College ) 0354-2254238 Darjeeling- 734101 14. St. Joseph’s College [1927] 0354-2270555/2270472 North Point-734 104 Surya Sen Mahavidyalaya [1998] 15. Surya Sen Colony, Block-B, Siliguri- 0353-2691488 734004 Ghoom Jorbungalow Degree College 16. [2005] (self-financed) 0354-2275130 Senchal Road, P.O. Ghoom- 734102 Gyan Jyoti College [2005] (self-finanecd) 17. 0353-2514087/ Dagapur (Pintail Village) NH-55, Hill Cart Road, 2005750/6531161 Siliguri- 734003 18. Nakshalbari College [2008] 0353 2005231 P.O. Nakshalbari- 734429 0353 2488044 Munshi Premchasd Mahavidyalaya 19. -

Education General Sc St Obc(A) Obc(B) Ph/Vh Total Vacancy 37 44 19 16 17 4 137 General
EDUCATION GENERAL SC ST OBC(A) OBC(B) PH/VH TOTAL VACANCY 37 44 19 16 17 4 137 GENERAL Sl No. University College Total 1 RAJ NAGAR MAHAVIDYALAYA 1 BURDWAN UNIVERSITY 2 SWAMI DHANANJAY DAS KATHIABABA MV, BHARA 1 3 AZAD HIND FOUZ SMRITI MAHAVIDYALAYA 1 4 BARUIPUR COLLEGE 1 5 DHOLA MAHAVIDYALAYA 1 6 HERAMBA CHANDRA COLLEGE 1 7 CALCUTTA UNIVERSITY MAHARAJA SRIS CHANDRA COLLEGE 1 8 SERAMPORE GIRLS' COLLEGE 1 9 SIBANI MANDAL MAHAVIDYALAYA 1 10 SONARPUR MAHAVIDYALAYA 1 11 SUNDARBAN HAZI DESARAT COLLEGE 1 12 GANGARAMPUR COLLEGE 1 13 GOURBANGA UNIVERSITY NATHANIEL MURMU COLLEGE 1 14 SOUTH MALDA COLLEGE 1 15 DUKHULAL NIBARAN CHANDRA COLLEGE 1 16 HAZI A. K. KHAN COLLEGE 1 17 KALYANI UNIVERSITY NUR MOHAMMAD SMRITI MAHAVIDYALAYA 1 18 PLASSEY COLLEGE 1 19 SUBHAS CHADRA BOSE CENTENARY COLLEGE 1 20 CLUNY WOMENS COLLEGE 1 21 LILABATI MAHAVIDYALAYA 1 NORTH BENGAL UNIVERSITY 22 NAKSHALBARI COLLEGE 1 23 RAJGANJ MAHAVIDYALAYA, RAJGANJ 1 24 ARSHA COLLEGE 1 SIDHO KANHO BIRSA UNIVERSITY 25 KOTSHILA MAHAVIDYALAYA 1 26 BHATTER COLLEGE 1 27 GOURAV GUIN MEMORIAL COLLEGE 1 28 PANSKURA BANAMALI COLLEGE 1 VIDYASAGAR UNIVERSITY 29 RABINDRA BHARATI MAHAVIDYALAYA 1 30 SIDDHINATH MAHAVIDYALAYA 1 31 SUKUMAR SENGUPTA MAHAVIDYALAYA 1 32 BARRAKPORE RASHTRAGURU SURENDRANATH COLLEGE 1 33 KALINAGAR MV 1 34 MAHADEVANANDA MAHAVIDYALAYA 1 WEST BENGAL STATE UNIVERSITY 35 NETAJI SATABARSHIKI MAHABIDYALAYA 1 36 P.N.DAS COLLEGE 1 37 RISHI BANKIM CHANDRA COLLEGE FOR WOMEN 1 OBC(A) 1 HOOGHLY WOMEN'S COLLEGE 1 BURDWAN UNIVERSITY 2 KABI JOYDEB MAHAVIDYALAYA 1 3 GANGADHARPUR MAHAVIDYALAYA -
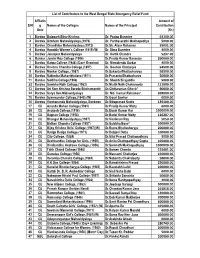
S/N Affliatin G Univ Names of the Colleges Names of the Principal Amount of Contribution (Rs) 1 Bardwa Balagarh Bijoy Krishna Dr
List of Contributors to the West Bengal State Emergency Relief Fund Affliatin Amount of S/N g Names of the Colleges Names of the Principal Contribution Univ (Rs) 1 Bardwa Balagarh Bijoy Krishna Dr. Pratap Banerjee 64100.00 2 Bardwa Birbhum Mahavidyalaya,(1979) Dr. Parthasarathi Mukhopadhya 5000.00 3 Bardwa Chandidas Mahavidyalaya,(1972) Dr.Sk. Ataur Rahaman 39001.00 4 Bardwa Hooghly Women's College (1919)(W) Dr. Sima Banerjee 4000.00 5 Bardwa Jamalpur Mahavidyalaya Dr. Kartik Chandra 5237.00 6 Bankur Jamini Roy College (1986) Dr.Pradip Kumar Banerjee 260000.00 7 Bardwa Katwa College.(1948) (Day+ Evening) Dr. Nirmalendu Sarkar 4000.00 8 Bardwa Krishna Chandra College,(1897) Dr. Goutam Chatterjee 54500.00 9 Bardwa Mankar College, (1987) Dr.Sukanta Bhattacharyya 58500.00 10 Bardwa Rabindra Mahavidyalaya (1971) Dr.Prasanta Bhattacharya 50000.00 11 Bankur Saldiha College(1966) Dr. Shaikh Sirajuddin 5000.00 12 Bardwa Sambhu Nath College,(1963) Dr.Nisith Nath Chakravorty 111000.00 13 Bardwa Sri Ram Krishna Sarada Sikshamandir Dr.Chittaranjan Ghosh* 90000.00 14 Bardwa Surya Sen Mahavidyalaya Dr. Md. Inamur Rahaman* 208000.00 15 Bardwa Syamsundar College,(1948) (W) Dr.Gouri Sankar 6000.00 16 Bardwa Vivekananda Mahavidyalaya, Burdwan Dr.Sibaprasad Rudra 139100.00 17 CU Ananda Mohan College,(1961) Dr.Pradip Kumar Maity 6000.00 18 CU Asutosh College,(1916) Dr.Dipak Kumar Kar 1000000.00 19 CU Bagnan College (1958) Dr.Badal Kumar Maity 116287.00 20 CU Bhangar Mahavidyalaya,(1997) Dr.Virvikram Roy 5050.00 21 CU Bidhan Chandra College (1957) Dr.Sulekha -

Advt. No. 1/2018)
THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status for the Posts of Assistant Professor in Government-aided Colleges of West Bengal (Advt. No. 1/2018) The Principal/Vice-Principal/Teacher-in-Charge of the Government-aided College of West Bengal are requested to check the Vacancy status (attached herewith) for the Post of Assistant Professor in the following subject and discrepancy detected, if any, please bring it to the notice of the office of the WBCSC within 5 days. Subject GEOGRAPHY Date : 06/03/2020 Controller of Examinations THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status for the Posts of Assistant Professor in Government-aided Colleges of West Bengal (Advt. No. 1/2018) GEOGRAPHY Sl No College University UR 1 Hiralal Bhakat College 2 Netaji Mahavidyalaya 3 Polba Mahavidyalaya BURDWAN UNIVERSITY 4 Polba Mahavidyalaya 5 Rampurhat College 6 Sagar Mahavidyalaya 7 Serampore Girls' College CALCUTTA UNIVERSITY 8 Sundarban Mahavidyalaya 9 Bakshirhat Mahavidyalaya CPB UNIVERSITY 10 Balurghat Mahila Mahavidyalaya 11 Pakuahat Degree College GOUR BANGA UNIVERSITY 12 Pakuahat Degree College 13 Dukhulal Nibaran Chandra College 14 Krishnath College KALYANI UNIVERSITY 15 Prof. Syed Nurul Hasan College 16 Banwarilal Bhalotia College, Asansol 17 Banwarilal Bhalotia College, Asansol KAZI NAZRUL UNIVERSITY (ASANSOL) 18 Banwarilal Bhalotia College, Asansol 19 Birpara College 20 Mirik College 21 Mirik College NORTH BENGAL UNIVERSITY 22 Saheed Kshudiram College 23 Sukanta Mahavidyalaya 24 University B.T. and Evening College 25 Manbhum -

Session 2020-2021
NSS ACTIVITIES AND PARTICIPATION SESSION 2020-2021 College: Bidhan Chandra College Principal: Dr. Falguni Mukhopadhyay Programme Officer: Dr. Barnali Pramanik (Unit– I) 1. PRE-REPUBLIC DAY PARADE CAMP AT BHUBANESWAR Venue Date No. of volunteers participated Bhubaneswar 22nd Nov – 01st Dec’20 1 NAME OF THE PARTICIPANT: RIDDHI PRASAD NAME OF SELECTED VOLUNTEERS FOR EAST ZONE PRE REPUBLIC DAY PARADE CAMP 2020 SERIAL NAME OF VOLUNTEER FATHER'S NAME GENDER DATE OF HEIGHT NAME OF UNIVERSITY NAME OF INSTITUION CONTACT NO EMAIL ID SPECIAL AREA (Cultural/ NO BIRTH (CM) (WhatsApp ) Parade) DD/MM/YY 1 LATE NARAYAN MALE 26/01/1997 170 CALCUTTA UNIVERSITY 7063388812 [email protected] MRIDUL BARMAN CALCUTTA UNIVERSITY PARADE CHANDRA BARMAN 2 SUMON ACHARYA TARUN ACHARYA MALE 06/10/1999 168 CALCUTTA UNIVERSITY SERAMPORE COLLEGE 8240589066 acharyasumon@gmailcom PARADE 3 MALE 2/26/1998 176 8240672069 [email protected] ANIKET DUTTA TARUN ACHARYA WEST BENGAL STATE UNIVERSITY DUM DUM MOTIJHEEL COLLEGE RECITATION 4 SHAHIN AKHTAR MALE 02/12/2001 180 6294058089 [email protected] APU DUTTA WEST BENGAL STATE UNIVERSITY BASIRHAT COLLEGE MONDAL 5 MALE 15/11/2001 177 COOCH BEHAR PANCHANAN BARMA 7872696958 [email protected] TANMOY DEBNATH NASIRUDDIN MONDAL TUFANGANJ MAHAVIDYALAYA PARADE UNIVERSITY 6 SOURAV SAHIS UTPAL DEBNATH MALE 07/08/2000 168 S.K.B.UNIVERSITY M.G COLLEGE,LALPUR 9531772883 [email protected] PARADE 7 GHATAK CHANDRA MALE 10/11/2000 168 7872732832 [email protected] SHIB NATH SING SARDAR S.K.B.UNIVERSITY -

THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status (Tentative) for the Posts of Assistant Professor in Government-Aided Colleges of West Bengal (Advt
THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status (Tentative) for the Posts of Assistant Professor in Government-aided Colleges of West Bengal (Advt. No. 1/2018) English UR OBC-A OBC-B SC ST PWD 46 15 20 25 36 16 Sl No College University UR 1 Birsa Munda Memorial College 2 Gobinda Prasad Mahavidyalaya 3 Jamini Roy College BANKURA UNIVERSITY 4 Patrasayer Mahavidyalaya 5 Raipur Block Mahavidyalaya 6 Ramananda College. (Day+ Evening) 7 Kandra Radhakanta Kundu Mahavidyalaya 8 Memari College 9 Purbasthali College BURDWAN UNIVERSITY 10 Sailajananda Falguni Smriti Mahavidyalaya 11 Syamsundar College 12 Gokhale Memorial Girls' College 13 Naba Ballygunge Mahavidyalaya CALCUTTA UNIVERSITY 14 Rammohan College 15 Udaynarayanpur Madhabilata Mahavidyalaya 16 Baneswar Sarathibala Mahavidyalaya CPB UNIVERSITY 17 Tufangunj College 18 Balurghat College 19 Dewan Abdul Gani College 20 Dr. Meghnad Saha College GOUR BANGA UNIVERSITY 21 Harishchandrapur College 22 Malda College 23 Berhampur College 24 Domkal Girls' College KALYANI UNIVERSITY 25 Nagar College 26 Prof. Syed Nurul Hasan College 27 Deshbandhu Mahavidyalaya KAZI NAZRUL UNIVERSITY (ASANSOL) 28 Falakata College 29 Falakata College 30 Ghoshpukur College 31 Kalimpong College NORTH BENGAL UNIVERSITY 32 Nani Bhattacharya Smarak Mahavidyalaya 33 Rajganj College 34 Sukanta Mahavidyalaya 35 University B.T. and Evening College 36 Bikramjeet Goswami Memorial College 37 Chitta Mahato Memorial College SIDHO KANHO BIRSHA UNIVERSITY 38 Panchakot Mahavidyalaya 39 Belda College 40 Chaipat Saheed Pradyot -

The West Bengal College Service Commission
THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status for the Posts of Assistant Professor in Government-aided Colleges of West Bengal (Advt. No. 1/2018) The Principal/Vice-Principal/Teacher-in-Charge of the Government-aided College of West Bengal are requested to check the Vacancy status (attached herewith) for the Post of Assistant Professor in the following subject and discrepancy detected, if any, please bring it to the notice of the office of the WBCSC within 5 days. Subject MATHEMATICS Date : 13/09/2019 Controller of Examinations THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status for the Posts of Assistant Professor in Government-aided Colleges of West Bengal (Advt. No. 1/2018) MATHEMATICS Sl No College University UR 1 Khatra Adibasi Mahavidyalaya. BANKURA UNIVERSITY 2 Panchmura Mahavidyalaya. 3 Abhedananda Mahavidyatan 4 Aghore Kamini Parkash Chandra Mahavidyalaya 5 Dr. Bhupendranath Dutta Smriti Mahavidyalaya BURDWAN UNIVERSITY 6 Guskara Mahavidyalkaya 7 Netaji Mahavidyalaya 8 Raja Ram Mohon Roy Mahavidyalaya 9 Harimohan Ghosh College 10 Purash-Kanpur Haridas Nandi Mahavidyalaya 11 Serampore Girls' College CALCUTTA UNIVERSITY 12 Shyampur Siddheswari Mahavidyalaya 13 Swami Niswambala Nanda Girls' College 14 Vijaygarh Jyotish Ray College 15 Dr. Meghnad Saha College GOUR BANGA UNIVERSITY 16 Berhampur Girls' College 17 Dukhulal Nibaran Chandra College 18 Jangipur College KALYANI UNIVERSITY 19 Krishnath College 20 Srikrishna College 21 Banwarilal Bhalotia College, Asansol 22 Banwarilal Bhalotia College, Asansol 23 Banwarilal -

Correction of NAD Registered Data. Dr. Debasis Dutta
1/21/2020 UNIVERSITY OF NORTH BENGAL Mail - Correction of NAD Registered data. nbuce nbuce <[email protected]> Correction of NAD Registered data. 1 message nbuce nbuce <[email protected]> Tue, Jan 21, 2020 at 5:23 PM To: "A.P.C. Roy Govt. College" <[email protected]>, AC College of Commerce <[email protected]>, Alipurduar College <[email protected]>, Alipurduar Mahila Mahavidyalaya <[email protected]>, Ananda Chandra College <[email protected]>, "Banarhat Kartick Orgaon Hindi Govt. College" <[email protected]>, Bijanbari Degree College <[email protected]>, Birpara College <[email protected]>, Birsa Munda College <[email protected]>, Chopra Kamal Paul Smriti Mahavidyalaya <[email protected]>, Cluny Women's College <[email protected]>, "Darjeeling Govt. College" <[email protected]>, Dhupguri Girls College <[email protected]>, Falakata College <[email protected]>, Ghoom Jorebunglow Degree College <[email protected]>, Ghoshpukur College <[email protected]>, "Gorubathan Govt. General Degree college" <[email protected]>, Gyanjyoti College <[email protected]>, Islampur College <[email protected]>, "Kalimpong College R.P. Dhakal" <[email protected]>, Kalipada Ghosh Tarai Mahavidyalaya <[email protected]>, Kurseong College <[email protected]>, Lilabati Mahavidyalaya <[email protected]>, Maynaguri College <[email protected]>, Mirik Degree College <[email protected]>, Munshi Premchand -

THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status (Tentative) for the Posts of Assistant Professor in Government-Aided Colleges of West Bengal (Advt
THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status (Tentative) for the Posts of Assistant Professor in Government-aided Colleges of West Bengal (Advt. No. 1/2018) CHEMISTRY UR OBC-A OBC-B SC ST PWD 36 8 6 26 21 6 Sl No College University UR 1 Abhedananda Mahavidyalaya 2 Kalna College BURDWAN UNIVERSITY 3 Polba Mahavidyalaya 4 Rampurhat College 5 Gour Mohan Sachin Mandal Mahavidyalaya CALCUTTA UNIVERSITY 6 Coochbehar College CPB UNIVERSITY 7 Dinhata College 8 Kaliachak College GOUR BANGA UNIVERSITY 9 Malda College 10 Dukhulal Nibaran Chandra College 11 Dukhulal Nibaran Chandra College 12 Dumkal College 13 Krishnath College KALYANI UNIVERSITY 14 Santipur College 15 Srikrishna College 16 Sripat Singh College 17 Kulti College KAZI NAZRUL UNIVERSITY (ASANSOL) 18 Triveni Devi Bhalotia College 19 Alipurduar College 20 Ananda Chandra College 21 Kalimpong College NORTH BENGAL UNIVERSITY 22 Kurseong College 23 Parimal mitra smriti mahavidyalaya 24 Sukanta Mahavidyalaya 25 Manbhum Mahavidyalaya 26 Raghunathpur College SIDHO KANHO BIRSHA UNIVERSITY 27 Raghunathpur College 28 Belda College 29 Belda College 30 Kharagpur College VIDYASAGAR UNIVERSITY 31 Midnapore College 32 Panskura Banamali College 33 Silda Chandra Sekhar College 34 Gobardanga Hindu College 35 Mrinalini Datta Mahavidyapith WEST BENGAL STATE UNIVERSITY 36 Saheed Nurul Islam Mahavidyalaya Sl No College University OBC-A 1 Saldiha College BANKURA UNIVERSITY 2 Harimohan Ghosh College CALCUTTA UNIVERSITY 3 Ramsaday College 4 Krishnagar Women's College KALYANI UNIVERSITY 5 Alipurduar -

Physico-Chemical Characterization and Biological Studies of Newly
J. Chem. Sci. (2018) 130:9 © Indian Academy of Sciences https://doi.org/10.1007/s12039-017-1409-9 REGULAR ARTICLE Physico-chemical characterization and biological studies of newly synthesized metal complexes of an Ionic liquid-supported Schiff base: 1-{2-[(2-hydroxy-5-bromobenzylidene)amino]ethyl}-3- ethylimidazolium tetrafluoroborate SANJOY SAHAa,∗, GOUTAM BASAKb and BISWAJIT SINHAc aDepartment of Chemistry, Kalimpong College, Kalimpong, West Bengal 734 301, India bDepartment of Microbiology, Raiganj University, Raiganj, West Bengal 733 134, India cDepartment of Chemistry, University of North Bengal, Darjeeling, West Bengal 734 013, India E-mail: [email protected] MS received 31 August 2017; revised 22 November 2017; accepted 22 November 2017; published online 1 February 2018 Abstract. Co(II), Ni(II) and Cu(II) complexes of an ionic liquid-supported Schiff base 1-{2-[(2-hydroxy-5- bromobenzylidene)amino]ethyl}-3-ethylimidazolium tetrafluoroborate were synthesized and characterized by various analytical and spectroscopic methods such as elemental analysis, UV-Visible, FT-IR,1H NMR, ESI MS, molar conductance and magnetic susceptibility measurements. Based on the spectral studies, tetra coordinated geometry was proposed for the complexes and molar conductance of the complexes revealed their electrolytic nature. The synthesized Schiff base and its complexes were evaluated for in vitro antibacterial activities against Gram positive and Gram negative bacteria. The complexes along with the Schiff base showed very significant biological activity against the tested bacteria. Keywords. Ionic liquid-supported Schiff base; Co (II)complex; Ni (II)complex; Cu (II)complex; antibacterial activity. 1. Introduction the field of inorganic and material chemistry. 8,9 The con- cept of functionalized ionic liquid (FILs), by introducing Ionic liquids (ILs) are organic salts which have low melt- additional a functional group as a part of cation or anion, ing points below the boiling point of water and are stable has presently become a subject of interest. -
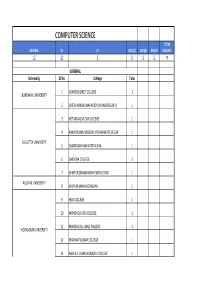
Computer Science Total General Sc St Obc(A) Obc(B) Ph/Vh Vacancy 17 31 7 9 9 0 73
COMPUTER SCIENCE TOTAL GENERAL SC ST OBC(A) OBC(B) PH/VH VACANCY 17 31 7 9 9 0 73 GENERAL University Sl No. College Total 1 ASANSOL GIRLS' COLLEGE 1 BURDWAN UNIVERSITY 2 VIVEKANANDA MAHAVIDYALAYA(HOOGHLY) 1 3 NETAJINAGAR DAY COLLEGE 1 4 RAMKRISHAN MISSION VIDYAMANDIR, BELUR 1 CALCUTTA UNIVERSITY 5 SAMMILANI MAHAVIDYALAYA 1 6 SARSUNA COLLEGE 1 7 SHIBPUR DINABANDHU INSTITUTION 1 KALYANI UNIVERSITY 8 KALYANI MAHAVIDYALAYA 1 9 HIJLI COLLEGE 1 10 MAHISADAL RAJ COLLEGE 1 11 MAHISHADAL GIRLS COLLEGE 1 VIDYASAGAR UNIVERSITY 12 PRABHAT KUMAR COLLEGE 1 13 RAJA N.L. KHAN WOMEN'S COLLEGE 1 14 VIVEKANANDA MISSION MAHAVIDYALAYA 1 15 BARASAT COLLEGE 1 WEST BENGAL STATE UNIVERSITY 16 DUM DUM MOTIJHEEL COLLEGE 1 17 MRINALINI DATTA MAHAVIDYAPITH 1 OBC(A) 1 RAJA RAMMOHAN ROY MAHAVIDYALAYA 1 BURDWAN UNIVERSITY 2 SRI RAMKRISHNA SARADA VIDYAMAHAPITHA 1 3 DHRUBA CHAND HALDER COLLEGE 1 CALCUTTA UNIVERSITY 4 JOGESH CHARDRA CHOUDHURI COLLEGE 1 5 RAMKRISHNA MISSION RESIDENTIAL COLLEGE 1 GOURBANGA UNIVERSITY 6 GOUR MAHAVIDYALAYA 1 7 CHANDRAKONA VIDYASAGAR MV 1 VIDYASAGAR UNIVERSITY 8 PANSKURA BANAMALI COLLEGE 1 9 VIVEKANANDA MISSION MAHAVIDYALAYA 1 OBC(B) 1 BANGABASI COLLEGE (DAY) 1 2 MAHESHTALA COLLEGE 1 CALCUTTA UNIVERSITY 3 NEW ALIPORE COLLEGE 1 4 NEW ALIPORE COLLEGE 1 NORTH BENGAL UNIVERSITY 5 SUKANTA MAHAVIDYALAYA 1 SIDHO KANHO BIRSA UNIVERSITY 6 PANCHAKOT MAHAVIDYALAYA 1 VIDYASAGAR UNIVERSITY 7 YOGODA SATSANGA PALPARA MAHAVIDYALAYA 1 8 BARRAKPORE RASHTRAGURU SURENDRANATH COLLEGE 1 WEST BENGAL STATE UNIVERSITY 9 PANIHATI MAHAVIDYALAYA 1 SC 1 ASANSOL GIRLS' COLLEGE 1 BURDWAN UNIVERSITY 2 MANKAR COLLEGE 1 3 MICHAEL MADHUSUDAN MEMORIAL COLLEGE 1 4 ANANDA MOHAN COLLEGE 1 5 ASUTOSH COLLEGE 1 6 ASUTOSH COLLEGE 1 7 BIDHAN CHANDRA COLLEGE(RISHRA) 1 CALCUTTA UNIVERSITY 8 CHARUCHANDRA COLLEGE 1 9 JOGESH CHARDRA CHOUDHURI COLLEGE 1 10 MAHESHTALA COLLEGE 1 11 RAMKRISHAN MISSION VIDYAMANDIR, BELUR 1 12 SHYAMPUR SIDDHESWARI MAHVIDYALAYA 1 GOURBANGA UNIVERSITY 13 GOUR MAHAVIDYALAYA 1 KALYANI UNIVERSITY 14 SRIKRISHNA COLLEGE 1 15 A.C.