Lizards As Vectors of Human Salmonellosis by F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Mineral Industry of New Zealand in 2007
2007 Minerals Yearbook NEW ZEALAND U.S. Department of the Interior December 2009 U.S. Geological Survey THE MINERAL INDUS T RY OF NEW ZEALAND By Pui-Kwan Tse New Zealand had more than 600 identified mineral zinc, could potentially be economically feasible if technologies occurrences in 25 different types of mineral deposits. New and prices become favorable. Excluding the petroleum industry, Zealand’s mineral production included gold, iron sand, and the value of New Zealand’s mineral production of coal, metals, silver; such industrial minerals as aggregate (crushed stone and industrial minerals accounted for about 1% of the gross and gravel), building and dimension stone, clay, diatomite, domestic product (GDP). The total value of New Zealand’s feldspar, lime and limestone for agricultural and industrial uses, minerals and mineral fuel production accounted for about 2% magnesite, marble, phosphate rock, salt, sulfur, and zeolite; and of the GDP. During the fiscal year from July 2006 to June 2007, mineral fuels. New Zealand’s total exploration expenditure on minerals and The Crown Minerals Act 1991 and the Crown Minerals mineral fuels was $NZ732.3 million ($512.5 million), of which Amendment Act 2003 set the broad legislative policy for petroleum accounted for 95% of the total (Ministry of Economic the prospecting and exploring for and the mining of Crown- Development, 2007a, p. 14). owned (meaning Government-owned on behalf of all New Zealanders) minerals in New Zealand. The Ministry of Production Economic Development, through the Crown Minerals Group, is responsible for the overall management of all state-owned In 2007, production of such commodities as bentonite, clay, minerals in New Zealand. -

Alps 2 Ocean Cycle Trail Visitor Survey 2020
LEAP Research Report No. 52 Alps 2 Ocean Cycle Trail Visitor Survey 2020 Lena Mkwara David Simmons Geoffrey Kerr November 2020 Alps 2 Ocean Cycle Trail Visitor Survey 2020 Lena Mkwara David Simmons Geoffrey Kerr Land Environment and People Research Report Report No. 52 November 2020 ISSN 1172-0859 (Print) ISSN 1172-0891 (PDF) ISBN 978-0-86476-452-2 (Print) ISBN 978-0-86476-455-3 (PDF) Lincoln University, Canterbury, New Zealand Abstract This report presents the findings from a 2020 survey of Alps 2 Ocean Cycle Trail (A2O) cyclists. COVID-19 cancelled fieldwork before data collection was complete. The limited data indicate that cyclists are extremely satisfied with the A2O and associated services, and make substantial expenditures associated with their ride. The A2O was a strong attractant to cyclists, the large majority of whom would not have visited the districts in the absence of the trail. Keywords A2O Cycle Trail, tourist attractions, tourism spending, economic attribution model, Mackenzie District, Waitaki District Acknowledgements This project benefitted immensely from the contributions of others. We wish to thank the following people for their generosity and assistance. Waitaki District Council, Tourism Waitaki and Cycle Journeys for their guidance and support. Accommodation providers and visitor centre operators who assisted with the distribution of survey cards. Lincoln University colleagues who peer reviewed the survey instrument. Dr Sally Driml, University of Queensland, for peer review of the survey instrument and the economic attribution model. Dr Yvonne Mathews, University of Waikato/Waikato Regional Council, for structuring the interactive mapping inputs. Dr Bentry Mkwara for GIS mapping assistance. -

SECTION 6: Otematata to Kurow 44Km LAKE BENMORE FITNESS:Easy SKILL: Easy TRAFFIC: High GRADE: 3
LAKE BENMORE 44km SECTION 6: Otematata to Kurow LAKE BENMORE FITNESS:Easy SKILL: Easy TRAFFIC: High GRADE: 3 SAILORS CUTTING BENMORE DAM www.alps2ocean.com Loch Laird Rd Map current as of 24/9/13 Te Akatarawa Rd TE AKATARAWA WAITANGI STATION SH83 STATION Te Akatarawa Rd OTAMATAPAIO RIVER 6 LAKE AVIEMORE OTEMATATA KIRKLISTON RANGE Deep Stream Walking Track DEEP STREAM FISHERMANS BEND OTEMATATA RIVER AVIEMORE DAM SH83 LAKE WAITAKI WAITAKI DAM Old Slip Rd ST MARYS RANGE Awahokomo Rd HAKATARAMEA AWAKINO KUROWRIVER 7 SH82 LEVEL 1000 800 SH83 AORAKI/MOUNT COOK AORAKI/MOUNT LAKE OHAU LODGE LAKE OHAU 600 BRAEMAR STATION TWIZEL OMARAMA 400 OTEMATATA KUROW 200 DUNTROON OAMARU 0 0 20 40 60 80 100 120 140 160 180 200 220 240 260 280 300 N WAITAKI RIVER KUROW CREEK 0 1 2 3 4 5km KEY: Onroad Off-road trail Scale Picnic Area Otiake Road Grants Rd From Otematata, ride up Loch Laird TRAIL IS UNDER CONSTRUCTION. Gards Rd Road and over the massive Benmore Highlights: OTIAKE RIVER Hydro Dam [5.5km]. It’s a steep road up to the dam, so you may need to • Benmore Dam Special School Rd walk the last 800 metres. Follow the Te • Te Akatarawa Road Akatarawa Road along the margins OTEKAIEKE RIVER of Lake Aviemore to the Aviemore • Lake Aviemore Dam [30km]. After crossing the Dam • Deep Stream Walking Track the trail follows the main road to Lake Waitaki and the Waitaki Dam [38km], • Aviemore Dam then to Kurow. This section of trail has • Fishermans Bend an interesting hydro history with dams and project towns. -

Thealps 2 Ocean
The Alps 2 Ocean Cycle Trail From The Mountains To The Sea! 5 Days, 4 Nights Moderate Grade tour highlights tour cost: 2020 / 2021 NZD$1995 • Aqua coloured glacial rivers and lakes • Vast wide open expanses options & supplements • Rich in history Bike Hire: NZD$190 • Varied and exciting riding Electric Bike Hire: NZD$450 • Great company Single Supplement: NZD$475 • Experienced attentive guide Tuatara Tours does not require single travellers pay a tours run surcharge for travelling alone. We will arrange for you to November - April Starting in Christchurch share accommodation with another traveller of the same gender and if we can not match you up we will provide a Custom Groups: Options are available for this tour single room at no extra charge. If you prefer not to share, a single supplement is payable to guarantee your own room. The cost of the single supplement is listed above. Tuatara Tours NZ Ltd, PO Box 13544, Christchurch 8141, New Zealand Phone: New Zealand: 0800 377 378, Australia: 1 800 044 633, World: +64 3 962 3280 Email: [email protected], Web: www.tuataratours.co.nz 1 The Alps 2 Ocean Cycle Trail From The Mountains To The Sea! 5 Days, 4 Nights Moderate Grade the tour Explore the new Alps 2 Ocean Cycle Trail, which makes official partner some of the South Island’s most dramatic scenery accessible to cyclists. Tuatara Tours is proud to be in an official partnership with The New Zealand Cycle There is nearly 300 km of cycling from the tussocky alpine Trail. Mackenzie Country with its turquoise lakes, through rural towns to the Pacific Ocean in historic Oamaru. -

II~I6 866 ~II~II~II C - -- ~,~,- - --:- -- - 11 I E14c I· ------~--.~~ ~ ---~~ -- ~-~~~ = 'I
Date Printed: 04/22/2009 JTS Box Number: 1FES 67 Tab Number: 123 Document Title: Your Guide to Voting in the 1996 General Election Document Date: 1996 Document Country: New Zealand Document Language: English 1FES 10: CE01221 E II~I6 866 ~II~II~II C - -- ~,~,- - --:- -- - 11 I E14c I· --- ---~--.~~ ~ ---~~ -- ~-~~~ = 'I 1 : l!lG,IJfi~;m~ I 1 I II I 'DURGUIDE : . !I TOVOTING ! "'I IN l'HE 1998 .. i1, , i II 1 GENERAl, - iI - !! ... ... '. ..' I: IElJIECTlON II I i i ! !: !I 11 II !i Authorised by the Chief Electoral Officer, Ministry of Justice, Wellington 1 ,, __ ~ __ -=-==_.=_~~~~ --=----==-=-_ Ji Know your Electorate and General Electoral Districts , North Island • • Hamilton East Hamilton West -----\i}::::::::::!c.4J Taranaki-King Country No,", Every tffort Iws b«n mude co etlSull' tilt' accuracy of pr'rty iiI{ C<llldidate., (pases 10-13) alld rlec/oralt' pollillg piau locations (past's 14-38). CarloJmpllr by Tt'rmlilJk NZ Ltd. Crown Copyr(~"t Reserved. 2 Polling booths are open from gam your nearest Polling Place ~Okernu Maori Electoral Districts ~ lil1qpCli1~~ Ilfhtg II! ili em g} !i'1l!:[jDCli1&:!m1Ib ~ lDIID~ nfhliuli ili im {) 6m !.I:l:qjxDJGmll~ ~(kD~ Te Tai Tonga Gl (Indudes South Island. Gl IIlllx!I:i!I (kD ~ Chatham Islands and Stewart Island) G\ 1D!m'llD~- ill Il".ilmlIllltJu:t!ml amOOvm!m~ Q) .mm:ro 00iTIP West Coast lID ~!Ytn:l -Tasman Kaikoura 00 ~~',!!61'1 W 1\<t!funn General Electoral Districts -----------IEl fl!rIJlmmD South Island l1:ilwWj'@ Dunedin m No,," &FJ 'lb'iJrfl'llil:rtlJD __ Clutha-Southland ------- ---~--- to 7pm on Saturday-12 October 1996 3 ELECTl~NS Everything you need to know to _.""iii·lli,n_iU"· , This guide to voting contains everything For more information you need to know about how to have your call tollfree on say on polling day. -

(NZ) Ltd Macraes Gold Project Macraes Phase III
Oceana Gold (NZ) Ltd Macraes Gold Project Macraes Phase III Landscape and Visual Assessment Oceana Gold (NZ) Ltd Macraes Gold Project Macraes Phase III Landscape and Visual Assessment Prepared by David McKenzie, FNZILA Opus International Consultants Ltd Principal, Landscape Architecture Environmental 20 Moorhouse Avenue PO Box 1482, Christchurch Mail Centre, Christchurch 8140, New Zealand Reviewed by Peter Rough, FNZILA Telephone: +64 3 363-5400 Director - Rough & Milne Landscape Facsimile: +64 3 365-7858 Architects Ltd Date: April 2011 Reference: 3-89554.00 Status: Final © Opus International Consultants Limited 2011 OceanaGold: Macraes Phase III Landscape and Visual Assessment Contents 1 Introduction ................................................................................................................. 1 1.1 Purpose of Document ................................................................................................... 1 1.2 Background Information ................................................................................................ 1 1.3 Outline of Macraes Gold Project Phase III .................................................................... 2 2 Site Context and Landscape Description .................................................................. 4 2.1 Landscape Context ....................................................................................................... 4 2.2 Site Landscape ............................................................................................................ -

Oceanagold Corporation Annual R Eport 2008
OceanaGold Corporation Annual Report 2008 ‘08 HIGHLIGHTS INCREASED YEARLY GOLD SALES BY COMMISSIONED THE FRASERS UNDERGROUND TO 264,124 MINE IN JANUARY – THE 49% OUNCES COMPANY’S SECOND NEW COMPARED TO FY2007 GOLD MINE IN NEW ZEALAND IN THE PAST TWO YEARS ACHIEVED EBITDA (EARNINGS BEFORE INTEREST, TAXES, DEPRECIATION EXCEEDED DESIGN AND AMORTISATION BUT EXCLUDING GAIN/LOSS THROUGHPUT RATES AT ON UNDESIGNATED HEDGES) OF THE REEFTON OPERATION AND PROCESSED $66.1 MILLION MORE THIS COMPARES TO FY 2007 19% ORE EBITDA OF $8.7 MILLION THAN PLANNED IN 2008 ACHIEVED STRONG CASH COST IMPROVEMENT BY Q4 THROUGH COMPLETED OF THE INCREASED 60% BULK EARTHWORKS AND DETAILED GOLD PRODUCTION DESIGN FOR THE DIDIPIO AND A DECREASE IN GOLD & COPPER PROJECT CONSUMABLE COSTS TO ACHIEVE IN LUZON, PHILIPPINES BEFORE TEMPORARILY PLACING IT ON PER CARE & MAINTENANCE $532 OUNCE OceanaGold Corporation Corporate Office Level 5, 250 Collins Street Melbourne, Victoria, 3000 Australia PO Box 355, Flinders Lane Post Office Melbourne, Victoria, 3000 Australia T: +61 3 9656 5300 F: +61 3 9656 5333 E: [email protected] www.oceanagold.com Delivering on sustainable growth 02 Chairman and CEO’s Review 06 Financial Analysis 10 Assets at a Glance 12 Operations 14 Development 16 Exploration Profile 22 Sustainability 40 Our People OceanaGold Corporation (OceanaGold) is a significant Pacific Rim gold producer listed on the Toronto, Australian 42 Corporate Governance and New Zealand Stock Exchanges. With three operating 48 Financial Statements gold mines and a portfolio of assets located in New Zealand 72 Shareholder Information and the Philippines, the company is forecast to produce between 280,000 to 300,000 ounces of gold in 2009. -

Otematata to Kurow Highlights: Safety Notes: Trail Surfaces
LAKE BENMORE 400 OTEMATATA KUROW 300 40km SECTION 6: Otematata to Kurow 200 ELEVATION LAKE BENMORE Fitness: Easy • Skill: Easy • Traffic: High • Grade: 3 100 0 0 10 20 30 40 50 KM SAILORS CUTTING 6km BENMORE DAM Loch Laird Rd CHECK WEBSITE FOR UPDATES BRIAR GULLY CAMPGROUND 20km T e A 17km ka TE AKATARAWA OTAMATAPAIO RIVER ta Te Akatarawa Rd SH83 ra CAMPGROUND wa Rd WAITANGI OTEMATATA CAMPGROUND KIRKLISTON RANGE LAKE AVIEMORE Deep Stream Walking Track DEEP STREAM 28km FISHERMANS BEND OTEMATATA RIVER AVIEMORE DAM SH83 LAKE WAITAKI 36km WAITAKI DAM TRAIL GUARDIAN AVIEMORE DAM TO DUNTROON ST MARYS RANGE Awahokomo Rd HAKATARAMEA AWAKINO RIVERKUROW SH82 Mapwww.alps2ocean.com current as of 20/2/17 N 40km 0 1 2 3 4 5km SH83 KEY: Onroad Off-road trail Picnic Area Scale WAITAKI RIVER From Otematata, take the sealed KUROW CREEK pathway beside Loch Laird Road, then follow the gravel track beside the lake. Otiake Road Grants Rd This takes you through several camping areas. You then ride up Loch Laird Road Gards Rd OTIAKE RIVER onto the massive Benmore Hydro Dam 6km . It’s a steep road up to the dam, so you may need to walk the last 800 Special School Rd metres. Follow the Te Akatarawa Road along the margins of Lake Aviemore OTEKAIEKE RIVER to the Aviemore Dam 28km . After crossing the dam the trail follows State Highway 83 to Lake Waitaki and the Trail route and weather conditions Waitaki Dam 36km , then to Kurow. This subject to change. Please check section of trail has an interesting hydro website before daily departure. -

Mineral, Coal and Petroleum Resources: Production,Exploration and Potential
2.3 MINERAL, COAL AND PETROLEUM RESOURCES MINERAL, COAL AND PETROLEUM RESOURCES: PRODUCTION, EXPLORATION AND POTENTIAL Anthony B. Christie1, Richard G. Barker2 1 GNS Science, PO Box 30-368, Lower Hutt 5040, New Zealand 2 Consulting Geologist, PO Box 54-094, The Marina, Bucklands Beach, Auckland 2144, New Zealand ABSTRACT: New Zealand has been a signifi cant producer of minerals and coal since early European settlement in the mid-19th century, and of hydrocarbons since 1970. Current production consists of oil, gold and silver, high quality (bituminous) coal, ironsand and specialised industrial minerals such as halloysite china clay for export, and a range of minerals and rocks for domestic use that are fundamental to New Zealand’s economy and infrastructure. The latter include aggregate for road making and construction (concrete aggregate and cement), coal for use by industry and electricity generation, and limestone for agriculture, cement making, and industry. Small quantities of diatomite, dolomite, perlite, pumice, serpentinite, and zeolite are also produced mainly for domestic markets. Other commodities that have been produced in the past include antimony, chromium, copper, lead, manganese, mercury, phosphate, platinum, sulphur, tin, tungsten, and zinc. New Zealand has well-documented potential for the discovery of a wide range of minerals and of petroleum, both onshore and offshore. Exploration is essential for converting this resource potential to wealth-creating assets. The resource-related industries are signifi cant economic contributors, but New Zealand’s resource potential remains to be realised. Mineral resources are created by natural processes and are generally described as non-renewable, implying they are fi nite, but many minerals (metals in particular) have been produced for several thousand years. -

Bugle 465 17 July 2019.Pub
Published by Issue 465 Kurow Wednesday Information Centre as a service to our community 17 July 2019 The Friends of the Waitaki Valley School would like to thank everyone who supported our yearly quiz on Friday 28th June. Special thanks to Ross and Trish of the Kurow Hotel, Hamish Benny our quizmaster and Rachael Chilton our scorekeeper. We raised just over $1,100. We are now looking forward to our next event, Bloke of the Year, on August 10th. TAKING ORDERS… Log Splitter with operator and Chainsaw man available in the Kurow -Twizel area from Sunday 14th July to Saturday 20th July to cut and split logs for firewood. Chainsaw $52 per hour Log Splitter $35 per hour with 1 man $65 per hour with 2 men Phone David on 027 426 0416 03 434 0080 230 Thames Highway Oamaru TO BOOK A COMMUNITY CAR: Phone 027 282 0615 or 4360 950 WAITAKI VALLEY PHONE BOOKS: We will print as requested. These have space for phone numbers from those who have not contributed their listing. While the listing was free we do ask for $5.00 per printed copy. Eftpos available at the Info Centre. BUGLE PIECES: Have you ever written an article you’d like published, maybe a poem or even a story...please let us know, we’d be happy to consider your piece for the Kurow Bugle! We are loving all the pieces that we’ve been given so far and hope that you are too! GYM KEYS: Gym Keys are no longer available from the Kurow Information Centre, you can purchase these from Waitaki Valley School - 436 0660. -
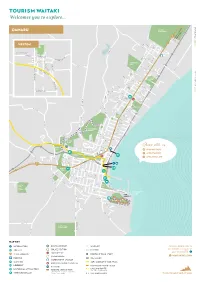
OAMARU M Oamaru a 1 RACECOURSE Ar H C Om R & U W CH 1 O T R IS
Tourism Waitaki D R N Welcomes you to explore... A W O G D R A 83 a OAMARU m Oamaru a 1 RACECOURSE ar H C Om R & U W CH 1 O T R IS U R #OurWaitaki K H C D o R T O H T T R To Quarry O W D IL FE E N RN WESTON V E B T SHARE I R K R S L E O D O S C K N B A R O S D E R D T I L M D R E R G R I D E O R V S D R E R E C Y S R E U LO To NGAPARA B V S A R I L L PL A AC S E T T MA S S IN ST C HARL E K ES ST R IC D O S T W T M IR R S R S L I I A Y HO D L N M EA G W N R EST S E E T D M C L A ARGYLE ST A S D T GR CENTENNIAL L E E R S PARK D O S H S S OMESTE A W T E AD N A S V RD D L R E D X I WH N S N E G T A H L S F A A LE M T E S C R V T T O ITEROC W E T B L O E A T S T L L ST M E S H T S G O E S VI D RA S N E R L T O W A ST O D W D R A T T T KS T S S E SP E R N R R D U R C C RD D E A MILNER A ST D W TE A AI S R TA PARK E UN KI M D A O W EL V VE H R S I A T RG E G IL LA S S N T H ST T AR O L E waitakinz.com FR C O H RE N M S G E T IN S A R T L ST N D BEDFORD W I T TH A S M T HBURN ST S R VISIT DISTRICT RD S C T TE A A L V D A IO R T E GH S ST OAKLEI CR T E S T T S S DO N E H N T S L I T C E O A D C L L C O E R N W DO N A Y NI A A ST N F W OR R D D O TH ET R T ST R R I DG C I K R O S A V T A&P R Y A E Showgrounds S W T E H L S T Y TU U G T R S I N H T F N I H O S L N Y Y L A S L T R E E D T E S W M T T T R A S S E H A E T D E D L Y L C ORANA W EA PARK VE L R A ST R K OR S W T ELL ST D T R S O S U N SE N S E T O D S E R A TRE P T NT S S E T K O DISTRICT RD T S GLEN WARREN T T N S S E N S RESERVE N S T E E R T M R OR A A R E IDG H XE E T S W T T S S -

Macraes Phase Iii Project Assessment of Economic
1 MACRAES PHASE III PROJECT ASSESSMENT OF ECONOMIC IMPACTS Mike Copeland Brown, Copeland & Co Ltd 14 April 2011 BLH-453174-226-635-V2 2 1. INTRODUCTION 1.1 Macraes Gold Project commenced operations in 1990 following the granting of initial consents in 1988. In 1992, 1997, 2000, 2001, 2002, 2004 and 2006 further consents were issued in relation to various expansions in both the rate of production and physical elements of the mine (pits, waste rock stacks and storage facilities for tailings and process water). In 2002 consent was granted for the development of a heritage and art park (HAP) on the site as part of the land rehabilitation package instead of the land reverting to pasture as initially intended. 1.2 OceanaGold (New Zealand) Limited is now seeking an extension to the consented life of the Macraes Gold Project. The extension called the Macraes Phase III Project will take the consented mine life through to 2020 or potentially longer, instead of the mine closing in 2012. The processing rate and intensity of operations on the site will be slightly higher than current levels, although production at the Fraser’s underground mine is expected to cease at the end of 2014 and current planning is for the Macraes opencast mining operations to be scaled back towards the end of the mine’s life (i.e. in 2019 and 2020). Should the mine no longer be operating after 2020 there will still be employment at the mine site for up to 18 months as rehabilitation works are undertaken. Environmental monitoring work would still be required on an intermittent basis.