Improving Decision Making in Intensive Care
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
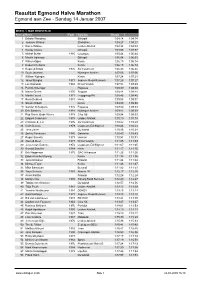
Resultat Egmond Halve Marathon Egmond Aan Zee - Søndag 14 Januar 2007
Resultat Egmond Halve Marathon Egmond aan Zee - Søndag 14 Januar 2007 MSEN, ½ MAR WEDSTRIJD PlassenNavn Født By/Club Brutto 1 Eshete Wondimu Ethiopië 1:04:14 1:04:14 2 Juwawo Wirimai Zimbabwe 1:04:27 1:04:27 3 Kamiel Maase Leiden Atletiek 1:04:34 1:04:34 4 Wellay Amare Ethiopië 1:04:58 1:04:57 5 Michel Butter 1985 Lycurgus 1:05:44 1:05:44 6 Mesfin Adimasu Ethiopië 1:06:04 1:06:03 7 Wilson Kigen Kenia 1:06:15 1:06:14 8 Robert Cheboror Kenia 1:06:15 1:06:14 9 Hugo vd Broek 1976 AV Castricum 1:06:23 1:06:22 10 Guus Janssen Nijmegen Atletiek 1:07:06 1:07:06 11 William Kiplagat Kenia 1:07:24 1:07:23 12 Jamal Baligha 1973 Improve Road Runners 1:07:28 1:07:27 13 Luc Krotwaar 1968 Prins Hendrik 1:07:51 1:07:49 14 Patrick Stitzinger Pegasus 1:08:39 1:08:38 15 Marco Gielen 1970 Scopias 1:08:44 1:08:44 16 Martin Lauret 1971 Loopgroep PK 1:08:46 1:08:45 17 Rens Dekkers 1981 Hera 1:09:08 1:09:07 18 Simion Ribish Kenia 1:09:09 1:09:08 19 Sander Schutgens 1975 Pegasus 1:09:40 1:09:39 20 Erik Sanders 1984 Nijmegen Atletiek 1:09:41 1:09:39 21 Rob Detert Oude Weme 1978 Ciko '66 1:09:54 1:09:53 22 Edgard Creemers 1975 Leiden Atletiek 1:10:19 1:10:18 23 Christian de Lie 1976 AV Castricum 1:10:22 1:10:20 24 Colin Bekers 1979 Loopteam Ed Sligcher 1:10:24 1:10:23 25 Timo Zeiler Duitsland 1:10:26 1:10:24 26 Serhiy Fiskovych 1980 Oekraïne 1:10:43 1:10:43 27 Roger Smeets 1973 Heerlen 1:10:51 1:10:51 28 Alex vd Meer 1977 Prins Hendrik 1:11:05 1:11:03 29 Jeroen van Damme 1972 Loopteam Ed Sligcher 1:11:07 1:11:05 30 Ronald Schröer 1984 Hera 1:11:17 1:11:15 31 Erik -

Hang De Poster Op!
het ledenmagazine van de politieke vereniging D66 Mei 2019 Democraat Vo or Europa Hang de poster op! p. 4 Aangenomen! p. 8 Even voorstellen… p. 24 Wouter Koolmees Ons programma voor de Ons (nieuwe) team Onze minister van Europese verkiezingen voor Europa Gelijke Kansen voorwoord 10. ondertussen in... Brussel wegnemen weer in de vingers krijgen, moet de hoogste prioriteit krijgen. Hoe we van Europa een continent maken dat zich niet van de ene naar de volgende crisis sleept, is de puzzel die 24. Democraten, nog een paar weken en dan Democraten, de enige echte Europa- we snel moeten gaan leggen. Hoe de Europese minister van gaan we, voor de tweede keer in korte tijd, naar partij van Nederland maakt zich op voor Unie weer een Unie wordt die zorgt voor haar Gelijke Kansen de stembus. Deze keer om ons jawoord aan Europese verkiezingen in een waar sleuteljaar. mensen, dat is het verhaal dat we de kiezer Europa te geven. Een overtuigd ‘ja’, voor een Op het moment van schrijven is het Verenigd moeten kunnen vertellen. democratisch, transparant en stabiel Europa, Koninkrijk nog lid van de Europese Unie, maar dat de wereld aankan. Om te kiezen voor de hoe en wanneer de Brexit afloopt is onduidelijk. In Zaandam hebben we daartoe een pro- 4. aangenomen! toekomst. Voor Europa. gramma aangenomen dat beoogt Europa fit te verkiezingscongres maken voor de toekomst. Voor een krachtig, In ons dagelijks leven, in gemeenten en provin- “ Onze Europese Unie verenigd Europa tussen de grote machtsblok- 8. onze top-10 voor Europa cies is Europa elke dag dichtbij. -

Esicm Annual Report 2019-2020 Contents
ESICM ANNUAL REPORT 2019-2020 CONTENTS I. Introduction by the President & President-Elect................................................................................................ 5 II. About ESICM............................................................................................................................................................6 1. Who we are......................................................................................................................................................... 9 2. Organisational Structure.................................................................................................................................11 3. Membership......................................................................................................................................................19 III. Actvities of the ESICM Division Of Scientific Affairs..........................................................................................22 Introduction....................................................................................................................................................... 24 1. ESICM Annual Congress & LIVES Forum....................................................................................................... 25 2. ESICM Research............................................................................................................................................... 29 Report on the Research Committee............................................................................................................... -

Oproep Aan Het Kabinet Kabinet: Toon Solidariteit in Europa, Ook Met Kinderen Op De Vlucht
OPROEP AAN HET KABINET KABINET: TOON SOLIDARITEIT IN EUROPA, OOK MET KINDEREN OP DE VLUCHT Griekenland stuurde zeven maanden geleden een noodkreet naar de lidstaten van de Europese Unie: Neem alstublieft samen een groep van 2.500 kinderen over die zonder familie vastzitten in de overvolle vluchtelin- genkampen. Deze kinderen - uit landen als Afghanistan en Syrië - zijn zeer kwetsbaar en hebben een veilige plek nodig. Deze noodkreet van de Griekse regering is door de coronacrisis alleen maar urgenter geworden. De situatie in de kampen is volgens artsen potentieel catastrofaal. Elf Europese landen hebben inmiddels gehoor gegeven aan de noodkreet van Griekenland. De evacuatie is begonnen en de eerste kinderen zijn vorige week overgebracht naar Luxemburg en Duitsland. Nederland moet zich solidair tonen en het voorbeeld van de andere EU lidstaten volgen. De tijd dringt en de situatie is urgent. WIJ ROEPEN HET KABINET OP OM - Een deel van deze groep kwetsbare kinderen op te vangen - Solidair te zijn met Griekenland en de andere landen die al meehelpen. BEVESTIGDE NAMEN • Dagmar Oudshoorn, directeur Amnesty International Nederland • Daan Roovers, Denker des Vaderlands • Abdelhuheb Choho, directeur Vluchtelingenwerk Nederland • Annemiek Schrijver, presentator KRO NCRV • Mirjam Blaak, directeur Defence for Children • Jos van Oord, theoloog, presentator KRO NCRV • Carel Kok, directeur Terre des Hommes • Frits de Lange, hoogleraar Ethiek Protestantse Theologische • Monique van ’t Hek, directeur Plan International Nederland Universiteit • Roeland Monasch, CEO Aflatoun • Mechteld Jansen, hoogleraar en rector Protestantse Theologische • Anna Timmerman, directeur PAX Universiteit Amsterdam-Groningen • Tineke Ceelen, directeur Stichting Vluchteling • Prof. Dr. Gerlach Cerfontaine, voorzitter VvAA • Michiel Servaes, directeur Oxfam Novib • Prof. -

Naam Instelling Prof.Ir. T. Asselbergs TU Delft Prof.Dr.Ir. C.Bakker TU Delft Dr
Naam Instelling Prof.ir. T. Asselbergs TU Delft Prof.dr.ir. C.Bakker TU Delft Dr. ir. Geert Aarts Wageningen University Jeroen Tjomme Alkema Wageningen University Andrew Allen Radboud University Eline Ampt Wageningen University Walther Ploos van Amstel Hogeschool van Amsterdam Muriel Arts Erasmus Universiteit M.A.L.M. van Assen Tilburg University Dr. Liesbeth Bakker NIOO-KNAW Julian Bakker Wageningen University Prof. Dr. Ir. Frank de Bakker IÉSEG School of Management Prof. dr. Ruud Balkenende TU Delft Dr. David Baneke Utrecht University Mariska Beekman Wageningen University Dr. P.J. Beers Erasmus Universiteit Jim van Belzen NIOZ Royal Netherlands Institute for Sea Research Rob van Bemmelen Wageningen University Prof. dr. Martin van den Berg Universiteit van Utrecht Prof. J. v.d. Bergh Universiteit Barcelona Dr. Arjen Biere NIOO-KNAW Prof. dr. F. Biermann Utrecht University Dr. J.D.L. van Bleijswijk NIOZ Royal Netherlands Institute for Sea Research Janneke Bloem Wageningen University Ir. Tess Blom TU Delft Prof. dr. P.M. van Bodegom Universiteit Leiden J.de Boer NIOO-KNAW Bertram de Boer Leiden University Prof.dr. J. Boersema Universiteit Leiden Margreet Boersma-de Jong Hanzehogeschool Dr. Aart Bontekoning Aart Bontekoning.nl Michiel P. Boom NIOO-KNAW Dr. Bram Bos wageningen university Dr.ir. Klaas Bouwmeester Wageningen University Jeffrey Brand MSc Utrecht University Prof. Dr. Ir. Han Brezet Aalborg Universiteit Prof. dr. Henk Brinkhuis Universiteit van Utrecht Ir. Ger Brinks Saxion Hogeschool Ir. Siebe Broersma TU Delft Jessica de Bruijn Wageningen University Prof. dr. Geert-Jan Brummer Vrije Universiteit Prof. dr. ir. B. Brunekreef Universiteit Utrecht professor E.M.van Bueren TU Delft Prof dr. -

Powerful Spin-Offs … Fostering Flexibility, Creativity and Individualised Critical Care!
Netherlands Journal of Critical Care Submitted April 2020; Accepted April 2020 EDITORIAL Powerful spin-offs … fostering flexibility, creativity and individualised critical care! D.W. Donker Department of Intensive Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands Correspondence D.W. Donker - [email protected] In recent weeks we have experienced the enormous impact of elements to maintain a high level of modern ventilatory care in the current coronavirus pandemic. Excellently guided by our these difficult times; rather, we should consider this ambition of society’s president, Diederik Gommers, we have all done our our young colleagues as a commendable example of how we can utmost as critical care professionals to respond in the best develop and implement novel elements of patient care within an possible ways to this crisis and we have succeeded in maintaining open-minded, self-improving critical care environment. a high level of patient care. Obviously, in this period, we feel the urgent need to quickly Above all, we are doing a great job by communicating effectively share our collective experience and all the latest insights on the and collaborating closely on a national and international level, coronavirus disease 2019 (COVID-19), as realised by the recent within our networks, our hospitals, units and teams. This is our webinar of the Dutch Society of Intensive Care moderated credo in the Netherlands intensive care networks, as recently by Iwan van der Horst. The link to the webinar can be found emphatically reflected by Verona Gerardu and Iwan van der at the end if this editorial. -

Ek, Die Voor Schiphol Wordt Gebruikt En Nu Dus Ook Voor Maastricht Aachen Airport
Eerste Kamer der Staten-Generaal 1 Vergaderjaar 2020–2021 35 205 Wijziging van de Wet belastingen op milieugrondslag (Wet vliegbelasting) D VERSLAG VAN EEN DESKUNDIGENBIJEENKOMST Vastgesteld 30 oktober 2020 De vaste commissie voor Financiën1 heeft op 6 oktober 2020 gesprekken gevoerd over: het SEO- en Districononderzoek naar de effecten van een vliegbelasting voor de luchtvrachtsector. Van deze gesprekken brengt de commissie bijgaand geredigeerd woordelijk verslag uit. De voorzitter van de commissie, Frentrop De griffier van de commissie, Van Dooren 1 Samenstelling: Essers (CDA), Koffeman (PvdD), Backer (D66), Ester (CU), Faber-van de Klashorst (PVV), Van Apeldoorn (SP), Sent (PvdA), Van Strien (PVV), Jorritsma-Lebbink (VVD), N.J.J. van Kesteren (CDA), Schalk (SGP), Van Rooijen (50PLUS), Adriaansens (VVD), Van Ballekom (VVD), Crone (PvdA), Frentrop (FVD), (voorzitter), Geerdink (VVD), Gerbrandy (OSF), Karimi (GL), (ondervoorzitter), Van der Linden (FVD), Otten (Fractie-Otten), Rietkerk (CDA), Rosenmöller (GL), Vendrik (GL), Van Wely (FVD) en Van der Voort (D66). kst-35205-D ISSN 0921 - 7371 ’s-Gravenhage 2020 Eerste Kamer, vergaderjaar 2020–2021, 35 205, D 1 Voorzitter: Frentrop Griffier: Van Dooren Via videoverbinding zijn aanwezig negen leden der Kamer, te weten: Atsma, Crone, Dessing, Frentrop, Geerdink, Van Strien, Teunissen, Vendrik en Van der Voort, alsmede: − de heer Stefan Grebe, CE Delft − de heer Jos Roeven, Maastricht Aachen Airport − de heer Pieter Elbers, KLM − de heer Steven Lak, Logistieke Alliantie − de heer Walter Manshanden, Netherlands Economic Observatory − de heer Thijs Boonekamp, SEO Economisch Onderzoek Aanvang 14.30 uur. De voorzitter: Alle sprekers zijn aanwezig. Ik heet de deskundigen van harte welkom. Ik vraag hun zo meteen om zich even voor te stellen. -

Oproep Aan Het Kabinet Kabinet: Toon Solidariteit in Europa, Ook Met Kinderen Op De Vlucht
OPROEP AAN HET KABINET KABINET: TOON SOLIDARITEIT IN EUROPA, OOK MET KINDEREN OP DE VLUCHT Griekenland stuurde zeven maanden geleden een noodkreet naar de lidstaten van de Europese Unie: Neem alstublieft samen een groep van 2.500 kinderen over die zonder familie vastzitten in de overvolle vluchtelingenkampen. Deze kinderen - uit landen als Afghanistan en Syrië ̈ - zijn zeer kwetsbaar en hebben een veilige plek nodig. Deze noodkreet van de Griekse regering is door de coronacrisis alleen maar urgenter geworden. De situatie in de kampen is volgens artsen potentieel catastrofaal. Elf Europese landen hebben inmiddels gehoor gegeven aan de noodkreet van Griekenland. De evacuatie is begonnen en de eerste kinderen zijn vorige week overgebracht naar Luxemburg en Duitsland. Nederland moet zich solidair tonen en het voorbeeld van de andere EU-lidstaten volgen. De tijd dringt en de situatie is urgent. WIJ ROEPEN HET KABINET OP OM - Een deel van deze groep kwetsbare kinderen op te vangen - Solidair te zijn met Griekenland en de andere landen die al meehelpen. NAMENS: (Update 23 april) • Neelie Kroes, oud-minister VVD en oud-EU-commissaris • Pieter Winsemius, oud-minister VVD • Tiny Kox, fractievoorzitter SP Eerste Kamer en fractievoorzitter Verenigd Links Raad van Europa • DWARS (GroenLinks) • ROOD (SP) • Marga van Praag, journalist en oud-presentator • Jacobine Geel, theologe en presentatrice KRO NCRV • Tim Hofman, presentator • Sinan Can, journalist • Hadewych Minis, actrice en zangeres • Art Rooijakkers, presentator • Theo de Roos, Hoogleraar strafrecht Universiteit van Tilburg • Prof. Ton Liefaard, hoogleraar kinderrechten, Universiteit Leiden • Prof. dr. Erik Scherder, hoogleraar klinische neuropsychologie • Prof. mr. Kees Groenendijk emeritus-hoogleraar rechtssociologie Radboud Universiteit Nijmegen • Geert Tom Heikens, PhD, MD, MSc. -
Netherlands Journal of Critical Care
Volume 28 - No 3 - May 2020 Netherlands Journal of Critical Care Bi-monthly journal of the Dutch Society of Intensive Care LETTER TO THE EDITOR EXPERT OPINION REVIEW Dedicated proning teams in the ICU Lung-protective mechanical ventilation in Delirium in the ICU – A structured review T.C. Roeleveld, H.J. de Vries, A. Girbes patients with COVID-19 of promising diagnostic and therapeutic H.J. de Vries, H. Endeman, approaches: Next steps in ICU delirium J.G. van der Hoeven, L.M.A. Heunks L. Smit, M. van der Jagt Netherlands Journal of Critical Care NETHERLANDS JOURNAL OF CRITICAL CARE EXECUTIVE EDITORIAL BOARD D.W. Donker, editor in chief CONTENTS I. van Stijn, managing editor H. Dupuis, language editor EDITORIAL D. van Dijk, associate editor M.M.J. van Eijk, associate editor 116 Powerful spin-offs … fostering flexibility, creativity and individualised N. Kusadasi, associate editor critical care! C.L. Meuwese, associate editor D.W. Donker [email protected] LETTER TO THE EDITOR COPYRIGHT 118 Dedicated proning teams in the ICU Netherlands Journal of Critical Care ISSN: 1569-3511 T.C. Roeleveld, H.J. de Vries, A. Girbes NVIC p/a Domus Medica P.O. Box 2124, 3500 GC Utrecht T: +31-(0)30 - 282 38 38 EXPERT OPINION 120 Lung-protective mechanical ventilation in patients with COVID-19 © 2020 NVIC. All rights reserved. Except as H.J. de Vries, H. Endeman, J.G. van der Hoeven, L.M.A. Heunks outlined below, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, REVIEW electronic, mechanical, photocopying, recording 126 Delirium in the ICU – A structured review of promising diagnostic and or otherwise, without prior written permission of the publisher. -

Overzicht Definitieve Kandidatenlijsten ______
_________________________________________________________________________________ Overzicht definitieve kandidatenlijsten _________________________________________________________________________________ Voor de verkiezing van: de Tweede Kamer der Staten-Generaal Dag van stemming: 17 maart 2021 Kieskring: Lelystad ________________________________________________________________________________ 1 VVD naam kandidaat woonplaats 1 Rutte, M. (Mark) (m) 's-Gravenhage 2 van Ark, T. (Tamara) (v) Nieuwerkerk aan den IJssel 3 Hermans, S.T.M. (Sophie) (v) Amsterdam 4 Becker, B. (Bente) (v) 's-Gravenhage 5 Yeşilgöz-Zegerius, D. (Dilan) (v) Amsterdam 6 van 't Wout, B. (Bas) (m) Hoeven 7 Harbers, M.G.J. (Mark) (m) Rotterdam 8 de Vries, A. (Aukje) (v) Leeuwarden 9 Wiersma, A.D. (Dennis) (m) Utrecht 10 Tellegen, O.C. (Ockje) (v) 's-Gravenhage 11 Aartsen, A.A. (Thierry) (m) Breda 12 Heinen, E. (Eelco) (m) 's-Gravenhage 13 Rajkowski, Q.M. (Queeny-Aimée) (v) Utrecht 14 Kamminga, R.J. (Roelien) (v) Groningen 15 El Yassini, Z. (Zohair) (m) Utrecht 16 van Campen, A.A.H. (Thom) (m) Zwolle 17 Paul, M.L.J. (Mariëlle) (v) Amsterdam 18 Erkens, S.P.A. (Silvio) (m) Kerkrade 19 Koerhuis, D.A.N. (Daniel) (m) Raalte 20 Michon-Derkzen, I.J.M. (Ingrid) (v) 's-Gravenhage 21 Valstar, P.J. (Peter) (m) 's-Gravenzande 22 Ellian, U. (Ulysse) (m) Almere 23 Van Wijngaarden, J. (Jeroen) (m) Amsterdam 24 de Groot, P.C. (Peter) (m) Harderwijk 25 van den Hil, J. (Jacqueline) (v) Goes 26 Minhas, F.B. (Fahid) (m) Schiedam 27 Tielen, J.Z.C.M. (Judith) (v) Utrecht 28 de Kort, A.H.J. (Daan) (m) Veldhoven 29 Heerema, R.J. (Rudmer) (m) Alkmaar 30 Brekelmans, R.P. (Ruben) (m) Oisterwijk 31 van Strien, P.J.T. -

Effective Use of Quality Indicators in Intensive Care
Effective use of quality indicators in intensive care Maartje L.G. de Vos Effective use of quality indicators in intensive care Maartje L.G. de Vos The research described in this thesis was carried out at the Department of Tranzo, Tilburg University, Tilburg, the Netherlands. The research was financially supported by the National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands. Cover design: Madelief Brandsma Cover photo: Ida Hylkema Printing: CPI Wöhrmann Print Service, Zutphen ISBN: 978-94-6203-163-0 This thesis was printed with financial support of: The National Intensive Care Evaluation (NICE) and Tilburg University. © Maartje de Vos, 2012 All rights reserved. No parts of this publication may be reproduced, stored, or trans- mitted in any way or by any means, without prior permission of the author. Effective use of quality indicators in intensive care PROEFSCHRIFT ter verkrijging van de graad van doctor aan Tilburg University op gezag van de rector magnificus, prof.dr. Ph. Eijlander, in het openbaar te verdedigen ten overstaan van een door het college voor promoties aangewezen commissie in de aula van de Universiteit op vrijdag 7 december 2012 om 14.15 uur door Maartje Louise Gerarda de Vos geboren op 13 december 1977 te Eindhoven Promotiecommissie Promotor: Prof. dr. G.P. Westert Copromotores: Dr. W.C. Graafmans Dr. P.H.J. van der Voort Overige leden: Prof. dr. D.H. de Bakker Prof. dr. D. Delnoij Prof. dr. J.G. van der Hoeven Prof. dr. E. de Jonge Dr. H.C.H. Wollersheim Contents Chapter 1 General -

Ontwikkelingen Rondom Het Coronavirus
maar toch even heel helder: wij werken als één team. 8 Eigenlijk met zijn vieren, ook met Tamara van Ark en Ferd Grapperhaus, en vaak met ondersteuning van de anderen Ontwikkelingen rondom het coronavi- die nodig zijn, zoals het economische trio Koolmees, Van rus 't Wout en Hoekstra, maar uiteraard ook de vicepremiers Kajsa Ollongren en Carola Schouten, en Arie Slob wat het Aan de orde is de voortzetting van het debat over de ont- onderwijs betreft. Zo sturen we met elkaar, echt als één wikkelingen rondom het coronavirus. team, deze crisis. We nemen met het gehele kabinet alle besluiten. De voorzitter: We gaan verder met het debat over de ontwikkelingen Dat betekent niet dat ik alle dossiers beheer. Het betekent rondom het coronavirus. We zijn toegekomen aan de ook niet dat ik alle portefeuilles overneem. Dat zou ook niks beantwoording van de zijde van het kabinet. Ik geef de helpen. Maar ik probeer op al die onderwerpen, of het nu minister-president het woord. gaat over het economische pakket, de maatschappelijke impact of alle zaken die de epidemiologie betreffen, zelf te bevorderen dat we de juiste besluitvorming hebben, dat er De beraadslaging wordt hervat. vragen gesteld worden, dat er onderwerpen voor geagen- deerd worden, dat iedereen aan de slag is en dat dingen die vastlopen, weer doorgaan. Dat betekent ook dat ik daar verantwoording over afleg aan de Kamer. En als er dingen Minister Rutte: niet goed gaan … Formeel ben ik geloof ik niet de baas in Dank u wel, voorzitter. Ik zou graag de vragen willen het Nederlandse staatsbestel, maar politiek ben ik dat beantwoorden die gaan over mijn rol bij het vaccinatiepro- natuurlijk in belangrijke mate wel.