Antigenicity of a Bacterially Expressed Triple Chimeric Antigen of Plasmodium Falciparum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 No. DSIR/MS/2019/02 Government of India Ministry of Science
No. DSIR/MS/2019/02 Government of India Ministry of Science & Technology Department of Scientific & Industrial Research MONTHLY SUMMARY FOR THE CABINET (For the month of February, 2019) (Part-I Unclassified) Ministry / Department : Department of Scientific and Industrial Research (DSIR) MAJOR ACHIEVEMENTS DURING THE MONTH OF FEBRUARY, 2019: DEPARTMENTAL ACTIVITIES 1. Industrial R&D Promotion Programme Recognition/ Registration and renewal of In-house R&D in Industry 21 in-house R&D units of industries were granted recognition as well as registration certificates. Scientific and Industrial Research Organization (SIROs) Recognition/ Registration and Renewal of SIROs 10 SIROs were granted recognition certificates. 84 SIROs were granted renewal of recognition certificates. Public Funded Research Institution (PFRIs) Registration and Renewal of PFRIs 04 PFRIs were granted renewal of registration. AUTONOMOUS BODY 1. Council of Scientific & Industrial Research (CSIR) 1.1 Hon’ble Prime Minister Confers Shanti Swarup Bhatnagar Prizes For Science and Technology Shri Narendra Modi, Hon‘ble Prime Minister and President CSIR Society conferred Shanti Swarup Bhatnagar (SSB) Prizes to 34 scientists in 7 disciplines, viz Biological Sciences; Chemical Sciences; Earth, Atmosphere Ocean and Planetary Sciences; Engineering Sciences; Mathematical Sciences; Medical Sciences and Physical Sciences for the year 2016, 2017 and 2018 at a special function organized by CSIR at Vigyan Bhawan on the occasion of National Science Day, celebrated on 28th of February. He said the theme for this year's National Science Day, which is, ‗Science for Society and Society for Science‘ is very relevant. Congratulating the award winners, the Prime Minister said that science, technology and innovation should relate to the aspirations and requirements of the society and try to find solutions for local problems. -
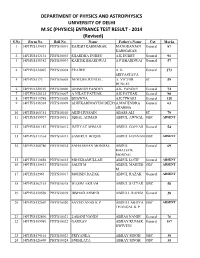
PHYSICS and ASTROPHYSICS UNIVERSITY of DELHI M.SC (PHYSICS) ENTRANCE TEST RESULT ‐ 2014 (Revised) S.No Form No
DEPARTMENT OF PHYSICS AND ASTROPHYSICS UNIVERSITY OF DELHI M.SC (PHYSICS) ENTRANCE TEST RESULT ‐ 2014 (Revised) S.No Form No. Roll No. Name Father's Name Cat. Marks 1 14PHYS139035 PHYS10001 RAJESH KARMAKAR MANORANJAN General 87 KARMAKAR 2 14PHYS152134 PHYS10002 SHARDHA DUBEY A K DUBEY General 91 3 14PHYS138382 PHYS10003 KARTIK BHARDWAJ A P BHARDWAJ General 57 4 14PHYS134007 PHYS10004 PRADHI A. K. General 173 SRIVASTAVA 5 14PHYS3173 PHYS10005 NEWLIFE RUNLEL A. VICTOR ST 59 RUNLEL 6 14PHYS129169 PHYS10006 ANIMESH PANDEY A.K. PANDEY General 74 7 14PHYS128121 PHYS10007 A NILAY PATHAK A.K.PATHAK General 90 8 14PHYS110748 PHYS10008 BHAWNA A.K.TIWARI General 128 9 14PHYS156301 PHYS10009 ADHIKARIMAYUM DEEPAA.MAHENDRA General 63 SHARMA 10 14PHYS160351 PHYS10010 ABID HUSSAIN ABASS ALI ST 79 11 14PHYS159937 PHYS10011 IQBAL AHMAD ABDUL AWWAL OBC ABSENT 12 14PHYS101187 PHYS10012 IMTIYAZ AHMAD ABDUL GAFFAR General 54 13 14PHYS113364 PHYS10013 SAMIRUL HOQUE ABDUL HANNAN OBC ABSENT 14 14PHYS168780 PHYS10014 SAHAJAHAN MONDAL ABDUL General 69 KHALECK MONDAL 15 14PHYS110658 PHYS10015 MD IKRAMULLAH ABDUL LOTIF General ABSENT 16 14PHYS135433 PHYS10016 SALIH M ABDUL MAJEED OBC ABSENT M 17 14PHYS12963 PHYS10017 MOHSIN RAZAK ABDUL RAZAK General ABSENT 18 14PHYS162161 PHYS10018 WASIM AKRAM ABDUL SATTAR OBC 58 19 14PHYS159509 PHYS10019 IRSHAD AHMED ABDULL RAHIM General 28 20 14PHYS132669 PHYS10020 SAYED ANAS K P ABDULLAKOYA OBC ABSENT THANGAL K P 21 14PHYS152890 PHYS10021 LABANI NANDI ABHAS NANDI General 76 22 14PHYS109989 PHYS10022 GAURAV ABHAY KUMAR General -

PM Confers Shanti Swarup Bhatnagar Prizes for S&T
PM confers Shanti Swarup Bhatnagar Prizes for S&T 01 March 2019 | News | By Manbeena Chawla The Shanti Swarup Bhatnagar (SSB) Prize for Science and Technology was instituted in the year 1957. Prime Minister Narendra Modi recently conferred Shanti Swarup Bhatnagar (SSB) prizes for the years 2016, 2017 and 2018 in New Delhi. The SSB prize is awarded each year on the basis of conspicuously important and outstanding contributions to human knowledge and progress, made through work done primarily in India during the five years, preceding the year of the prize. The SSB prize, comprising a citation, a cash award of Five Lakh rupees and a plaque is given to each person selected for the award in the following disciplines viz. Biological sciences, Chemical Sciences, Medical Sciences, Physical Sciences, Mathematical Sciences, Engineering Sciences and Earth, Atmosphere, Ocean and Planetary Science. The Shanti Swarup Bhatnagar (SSB) Prize for Science and Technology was instituted in the year 1957, in the memory of late Dr (Sir) Shanti Swarup Bhatnagar, the founder director of the Council of Scientific and Industrial Research(CSIR). Any citizen of India engaged in research in any field of science and technology up to the age of 45 years is eligible to be nominated. List of receipients in the field of Biological Sciences and Medical Sciences- Dr Rishikesh Narayanan, Indian Insitute of Science- 2016- Biological Sciences Dr Suvendra Nath Bhattacharya, Indian Institute of Chemical Biology- 2016- Biological Sciences Dr Niyaz Ahmed A S, University of Hyderabad- 2016- Medical Sciences Dr Deepak T Nair, Regional Centre of Biotechnology- 2017- Biological Sciences Dr Sanjeev Das, National Institute of Immunology- 2017- Biological Sciences Dr Amit Dutt, ACTREC, Tata Memorial Centre- 2017- Medical Sciences Dr Deepak Gaur, Jawaharlal Nehru University- 2017- Medical Sciences Dr Ganesh Nagaraju, Indian Insitute of Science- 2018- Biological Sciences Dr Thomas Pucadyil, Indian Institute of Science Education and Research- 2018- Biological Sciences Dr Ganesan V, NIMHANS- 2018- Medical Sciences . -
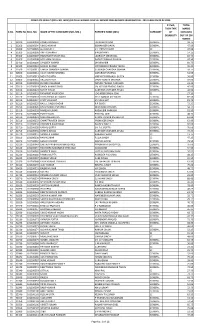
Result (Roll No
COMPLETE RESULT (ROLL NO. WISE) OF DELHI HIGHER JUDICIAL SERVICE PRELIMINARY EXAMINATION - 2019 HELD ON 02.02.2020 IF PwD, TOTAL THEN TYPE MARKS S.NO. FORM No. ROLL NO. NAME OF THE CANDIDATE (MR./MS.) FATHER'S NAME (MR.) CATEGORY OF OBTAINED DISABILITY OUT OF 200 MARKS 1 33100 101200002 PANKAJ PRABHAT JAGDISH PRASAD GENERAL 32.75 2 33200 101200004 VINOD KUMAR BISHAMBER DAYAL GENERAL 47.50 3 30300 101200005 GURUMUKH SH. PREM CHAND SC 47.00 4 31300 101200006 ANITA SHARMA B N SHARMA GENERAL 14.25 5 33300 101200007 HARVINDER SINGH GILL JAGROOP SINGH GILL GENERAL 82.75 6 31400 101200008 NEELMANI SHUKLA RADHEY RAMAN SHUKLA GENERAL 47.50 7 32400 101200009 SANDEEP KUMAR DHARAMBIR GENERAL 60.75 8 31500 101200010 NAWIN KUMAR VIDYANAND PRASAD YADAV GENERAL 45.25 9 33500 101200011 UMESH CHANDRA SAXENA SUBHASH CHANDRA SAXENA GENERAL 59.75 10 30600 101200012 VIJAY KUMAR SHARMA DATARAM SHARMA GENERAL 51.50 11 32600 101200013 MOHIT GUPTA VIRENDER PRAKASH GUPTA GENERAL 56.00 12 30800 101200015 ARUJ MATHUR VINAY KUMAR MATHUR GENERAL 59.00 13 31010 101200018 EUREKA CHAUHAN NANDU PRASAD CHAUHAN GENERAL 22.25 14 32010 101200019 SANOJ KUMAR SINGH LAL KISHOR PRASAD SINGH GENERAL 45.00 15 30110 101200021 SUMIT AHUJA SUBHASH CHANDER AHUJA GENERAL 42.50 16 31110 101200022 RAMANJIT KAUR SOHI JOGINDER SINGH SOHI SC 27.50 17 32110 101200023 SYED ZISHAN ALI WARSI SYED QAMAR ALI WARSI GENERAL 82.50 18 33110 101200024 JYOTI VASHISHT SN VASHISHT GENERAL 81.75 19 31210 101200025 RAHUL SINGH DAGAR R P SINGH GENERAL 72.25 20 33210 101200026 KHUSHVINDER SHARMA DEVENDER SHARMA GENERAL 58.75 21 31310 101200028 NARESH KUMAR RAGHUBIR PARKASH GENERAL 81.50 22 32310 101200029 PUNEET JAIN PINK RAJ JAIN GENERAL 48.75 23 32410 101200031 NIDHI MEHROTRA KAMAL KUMAR MEHROTRA GENERAL 62.00 24 31510 101200032 CHHATTRAVEER SINGH PREMVEER SINGH GENERAL 62.00 25 30710 101200034 HERINDER KAUR BRAR BALDEV SINGH GENERAL 92.00 26 31710 101200035 KAMAL GUPTA SH. -
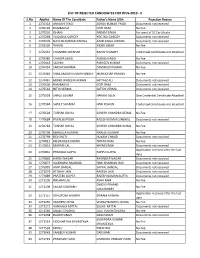
List of Rejected Candidate for the Post of H.P. Judicial Services Examination-2019-II 2028KB
LIST OF REJECTED CANDIDATES FOR HPJS-2019 - II S.No. Applno Name Of The Candidate Father's Name S/Sh. Rejection Reason 1 1270118 ANKUSH TYAGI ASHOK KUMAR TYAGI Documents not received 2 1270139 RAMBAKSH GOPI RAM No Fee 3 1270226 ISHANI PADAM SINGH For want of SC Certificate 4 1270268 YUGASHA GARGEY YOG RAJ GARGEY Documents not received 5 1270276 NAVEEN KUMAR AWANA AZAD SINGH AWANA Documents not received 6 1270302 VARUN INDER SINGH No Fee 7 1270341 CHANDRA SHEKHAR RAJESH PANDEY Credential Certificates not attached 8 1270380 CHHAYA JAGGI SUBASH JAGGI No Fee 9 1270443 SACHIN PARVEEN KUMAR Documents not received 10 1270454 SAKSHI SHARMA DAVINDER KUMAR No Fee 11 1270484 SHAILANDAR KUMAR PANDEY MURLIDHAR PANDEY No Fee 12 1270487 MOHD WASEEM AKRAM IMTIYAZ ALI Documents not received 13 1270536 RAMBAKSH GOPI RAM Documents not received 14 1270542 NITIN VERMA SATISH VERMA Documents not received 15 1270558 SHALU BEGAM HAKAM DEEN One Credential Certificate Attached 16 1270584 SHAILY SHARMA HARI KISHAN Credential Certificates not attached 17 1270628 TUSHAR GOYAL SURESH CHANDRA GOYAL No Fee 18 1270684 PAYAL KAPOOR RAJESH KUMAR SINGHAL Documents not received 19 1270718 TUSHAR GOYAL SURESH CHANDRA GOYAL No Fee 20 1270798 MANJULA KUMARI NARESH KUMAR No Fee 21 1270799 DEV JYOTI HUKAM CHAND Documents not received 22 1270812 MEENAKSHI KHATRI TIRATH RAM No Fee 23 1270819 MURARI LAL KHEM SINGH Documents not received Application received after the last 24 1270861 PRANJALI GUPTA PAPPU GUPTA date 25 1270865 HARSH NAGAR RAVINDER NAGAR Documents not received 26 1270874 -

Awardees of National Bioscience Award for Career Development
AWARDEES OF NATIONAL BIOSCIENCE AWARD FOR CAREER DEVELOPMENT Awardees for the year 2016 1. Dr. Mukesh Jain, Associate Professor, School of Computational and Integrative Sciences, Jawaharlal Nehru University, New Delhi-110067 2. Dr. Samir K. Maji, Associate Professor, Indian Institute of Technology, Powai, Mumbai- 400076 3. Dr. Anindita Ukil, Assistant Professor, Calcutta University, Kolkata 4. Dr. Arnab Mukhopadhyay, Staff Scientist V, National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi- 110067 5. Dr. Rohit Srivastava, Professor, Indian Institute of Technology, Bombay, Mumbai- 400076 6. Dr. Pinaki Talukdar, Associate Professor, Indian Institute of Science Education and Research, Dr. Homi Bhabha Road, Pashan, Pune- 7. Dr. Rajnish Kumar Chaturvedi, Senior Scientist, CSIR- Indian Institute of Toxicology Research, Lucknow-226001 8. Dr. Jackson James, Scientist E-II, Neuro Stem Cell Biology Lab, Neurobiology Division, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala- 695014 Awardees for the year 2015 1. Dr. Sanjeev Das, Staff Scientist-V, National Institute of Immunology, New Delhi 2. Dr. Ganesh Nagaraju, Assistant Professor, Department of Biotechnology, Indian Institute of Science, Bangalore- 5600012. 3. Dr. Suvendra Nath Bhattacharya, Principal Scientist, CSIR- Indian Institute of Chemical Biology, Kolkata- 700032 4. Dr. Thulasiram H V, Principal Scientist, CSIR-National Chemical Laboratory, Pune- 411008. 5. Dr. Pawan Gupta, Principal Scientist, Institute of microbial Technology, Chandigarh- 160036. 6. Dr. Souvik Maiti, Principal Scientist, CSIR-Institute of Genomics and Integrative Biology, Delhi- 110025. 7. Dr. Pravindra Kumar, Associate Professor, Department of Biotechnology, IIT, Roorkee- 247667. 8. Dr. Anurag Agrawal, Principal Scientist, CSIR-Institute of Genomics and Integrative Biology, Delhi- 110025 9. Dr. Gridhar Kumar Pandey, Professor, Department of Plant Molecular Biology, University of Delhi South Campus, New Delhi- 110067 10. -

Roll No Wise Marks List of Candidates for the Post of Astt. Accountant In
ROLLNO WISE MARKS LIST OF CANDIDATES FOR THE POST OF ASTT. ACCOUNTENT IN TREASURIES/SUB TREASURIES OF UTTARAKHAND DISTRICT : PAURI GARHWAL COMBINED Page No. 1 SL# Rollno Candidate's Name Father's Name D-O-B Catg. H.Catg. Marks 1 210001 VIKRAM SINGH NEGI SATYAPAL SINGH 08/12/1984 GEN GEN 112.00 2 210002 MANISH NAUTIYAL BHAGWATI PRASAD NAUTIYAL 14/12/1985 GEN GEN 79.50 3 210003 OM PRAKASH RAWAT GOVIND SINGH RAWAT 01/12/1986 OBC GEN 47.00 4 210005 BALBEER SINGH NEGI MUKAND SINGH NEGI 05/07/1976 GEN GEN 45.25 5 210007 KHILAP SINGH KESHAR SINGH DANU 21/03/1982 GEN GEN 92.00 6 210008 KULDEEP SINGH NEGI GAUMBHIR SINGH NEGI 18/07/1980 GEN GEN 78.50 7 210011 ABHAY PANDEY OM PRAKASH PANDEY 28/11/1988 GEN GEN 105.25 8 210012 SURENDRA SINGH SADAR SINGH 11/07/1982 GEN GEN 86.25 9 210014 GANESHPAL SINGH SRI JAIPAL SINGH 15/10/1981 GEN GEN 59.50 10 210016 MANISH KUMAR D C CHANDOLA 06/01/1978 GEN GEN 81.00 11 210017 SAPNA THAKUR ASHOK CHAND 15/12/1979 GEN WO 51.25 12 210018 ROHIT NEGI HARPAL SINGH NEGI 01/07/1985 GEN GEN 72.00 13 210019 SUNIL SINGH ROSHAN LAL 30/04/1983 SC GEN 34.25 14 210020 JITENDRA SINGH SHAILENDRA SINGH 05/02/1979 OBC GEN 42.00 15 210021 MANOJ KUMAR SHRI RAJENDRA PRASAD 01/01/1989 GEN GEN 37.75 16 210022 BHUWAN CHANDRA PANDEY HARISH CHANDRA PANDEY 08/01/1979 GEN GEN 107.00 17 210023 AJAY KUMAR BHARTI LATE MR GUDDU DAS 06/10/1985 SC GEN 54.75 18 210025 AJAY RAWAT BHAGWAT CHARAN RAWAT 25/08/1983 GEN GEN 27.75 19 210026 MANOJ KUMAR SRI SUFAL DASS 24/09/1975 SC GEN 62.50 20 210027 SURAJ NEGI TIRTH SINGH NEGI 22/06/1982 GEN GEN 45.75 21 210028 DEEPAK BISHT S S BISHT 22/08/1980 GEN GEN 50.75 22 210029 PAWAN KUMAR LAKHERA C B LAKHERA 03/01/1978 GEN GEN 60.00 23 210030 RAKESH ROSHAN PANT BASBANAND PANT 09/04/1982 GEN GEN 126.75 24 210031 RASHMI RAWAT UMESH RAWAT 18/04/1984 GEN WO 68.25 25 210032 SAURABH BISHT SHRI PUSHKER SINGH BISHT 30/07/1985 GEN GEN 96.00 26 210033 SUDHANSHU BHATT GIRISH CHANDRA BHATT 27/11/1986 GEN GEN 79.25 27 210034 GUNJAN RAUTELA RAMESH CHANDRA SINGH 18/03/1984 GEN WO 81.00 ROLLNO WISE MARKS LIST OF CANDIDATES FOR THE POST OF ASTT. -
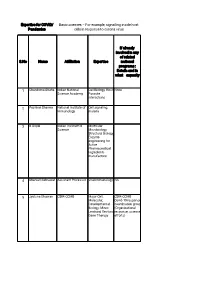
Basic Sciences – for Example, Signalling Inside Host Pandemics Cells in Response to Corona Virus
Expertise for COVID/ Basic sciences – For example, signalling inside host Pandemics cells in response to corona virus If already involved in any of related S.No Name Affiliation Expertise national programs : Details and in what capacity 1 Chandrima Shaha Indian National Cell Biology, Host None Science Academy Parasite interactions 2 Pushkar Sharma National Institute of Cell signaling, Immunology malaria 3 B Gopal Indian Institute of Molecular Science Microbiology, Structural Biology, Enzyme engineering for Active Pharmaceutical Ingredients manufacture 4 Sharvan Sehrawat Assistant Professor Viral immunology NA 5 Jyotsna Dhawan CSIR-CCMB Major-Cell, CSIR-CCMB Molecular, Covid-19 response Developmental coordination group Biology; Minor: (Organizational Lentiviral Vectors, response, science Gene Therapy efforts) 6 G. Baskaran The Institute of Theoretical Mathematical Physics. Sciences, Chennai Curious about 600 113 and biological IITMadras processes for decades. 7 Pradip Kumar Jamia Hamdard, NewMolecular NA Chakraborti Delhi 110062 Microbiology, Infectious disease, prokaryotic signalling 8 Nahid Ali CSIR Indian institute Parasitology, of chemical biology immunology 9 Raj Kumar Roy IISER Mohali Polymer and No Material Chemistry 10 Narinder K. Mehra All India Institute of COVID-19 and Medical Sciences immunity, Laboratory diagnosis 11 V. Ramanathan IIT(BHU) Varanasi Raman imaging Have been helping for disease the administrative diagnosis authorities of my city and my institute in making and distributing hand sanitizers. Till date have made around -

February 2019 Report
February 2019 India Science Wire - highlighting Indian science in Indian media The coverage of science and technology particularly relating to research done in Indian research institutions, is generally very poor in Indian media. There are several reasons for this situation, one of them being the lack of credible and relevant science content. In order to bridge this gap, Vigyan Prasar launched a unique initiative - India Science Wire (ISW) – in January 2017. The news service is dedicated to developments in Indian research laboratories, universities and academic institutions. Almost all news stories released by this service are based on research papers by Indian scientists published in leading Indian and foreign journals. All news stories and features are written and edited by a team of professional science journalists with decades of experience in science journalism. News stories based on happenings in Indian research labs are released to media houses on a daily basis. These stories are also uploaded on ISW website and are simultaneously promoted though social media – Twitter and Facebook. At present, the service is available in English and Hindi. Reach out ISW Editor with story ideas, comments and suggestions at [email protected] ISW website: http://vigyanprasar.gov.in/isw/isw.htm ISW stories released and published in Feubuary 2019 S.No Story title Date of Name of the writer release 1 New study says haze may be February 1 Dinesh C Sharma contributing to warming in South Asia 2 New material from silk protein and February 1 P Surat -
Biotechnology Logy
Biotechnology transformation socio-economic sustainable for agent An Biotechnology An agent for sustainable socio-economic transformation Biotechnology An agent for sustainable Special Volume on socio-economic transformation 30 Years of Department of Biotechnology, Ministry of Science & Technology, Government of India Supported by Special Volume on 30 Years of Department of Biotechnology, Ministry of Science & Technology, Government of India Media Partner Ministry of Science & Technology Government of India Global Biotechnology Summit Celebrating Biotechnology: Destination India 5th - 6th Feb, 2016 at Vigyan Bhawan, New Delhi www.dbtindia.gov.in Biotechnology: An agent for sustainable socio-economic transformation Biotechnology: An agent for sustainable socio-economic transformation Adventurous Thirty One of the key government departments of India www.nature.com/natureindia credited with heralding the era of adventure and entrepreneurship in the country’s science – the Department of Biotechnology (DBT) – is 3A, 5th Floor, DLF Corporate Park, M G Road Phase-3 Gurgaon 122002, Haryana, India entering its youthful years. Thirty, as they say, is Ph: +91 124 3079600/+91-124-3079657 the new twenty. In these three eventful decades, DBT’s achievements have been many, in terms EDITORIAL of the science it has enabled, the policies it Editor: Subhra Priyadarshini has introduced, as well as the linkages it has established across disciplines and countries. Copy Editor: Twarita Chakraborty DBT’s many forays into biological sciences Art and design: Ajay Kumar Bhatt education and its societal contributions have Cover image © PhotoDisc/Getty Images also bolstered its position as a formidable science-faring body in the country – as a PUBLISHING confident young global icon who looks to NPG India the future, armed with a dynamic policy biodiversity to big data. -
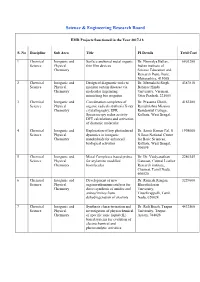
List of Projects Funded by SERB During 2017-18
Science & Engineering Research Board EMR Projects Sanctioned in the Year 2017-18 S. No Discipline Sub Area Title PI Details Total Cost 1 Chemical Inorganic and Surface anchored metal organic Dr. Nirmalya Ballav, 6803280 Science Physical thin film devices Indian institute of Chemistry Science Education and Research Pune, Pune, Maharashtra, 411008 2 Chemical Inorganic and Design of diagnostic tools to Dr. Meenakshi Singh, 4387810 Science Physical monitor certain diseases via Banaras Hindu Chemistry molecular imprinting University, Varanasi, mimicking bio recgnitor Uttar Pradesh, 221005 3 Chemical Inorganic and Coordination complexes of Dr. Prasanta Ghosh, 4163280 Science Physical organic radicals systhesis X-ray Ramakrishna Mission Chemistry crystallography, EPR Residential College, Spectroscopy redox activity Kolkata, West Bengal, DFT calculations and activation of diatomic molecular 4 Chemical Inorganic and Exploration of key photinduced Dr. Samir Kumar Pal, S 1998000 Science Physical dyanmics in inorganic N Bose National Centre Chemistry nanohybrids for enhanced for Basic Sciences, biological activities Kolkata, West Bengal, 700098 5 Chemical Inorganic and Metal Complexes based probes Dr. Dr. Vaidyanathan 2286145 Science Physical for arylamine modified Ganesan, Central Leather Chemistry biomlecules Research institute, Chennai, Tamil Nadu, 600020 6 Chemical Inorganic and Development of new Dr. Ramesh Rengan, 3229600 Science Physical organoruthenium catalysis for Bharathidasan Chemistry direct synthesis of amides and University, anines/imines from Tiruchirappalli, Tamil dehydrogenation of alcohols Nadu, 620024 7 Chemical Inorganic and Synthesis characterization and Dr. Ruli Borah, Tezpur 4432560 Science Physical investigation of physiochemical University, Tezpur, Chemistry of specific ionic liquid (IL) Assam, 784028 based systems for evalution of electrochemical and photocatalytic activities 8 Chemical Inorganic and A Comparative study on Dr. -

CSIR News Newsletter of the Council of Scientific & Industrial Research
ISSN 0409-7467 CSIR News NEWSLETTER OF THE COUncIL OF SCIENTIFIC & INDUSTRIAL RESEARCH Volume 69 No. 3 & 4 website: http://www.csir.res.in February 2019 In The News Prime Minister Narendra Modi Confers Shanti Swarup Bhatnagar Awards for S&T ON the occasion of the National Science Day, 2017 and 2018 for Science and Technology to the Prime Minister Shri Narendra Modi conferred the awardees in an award ceremony held at Vigyan Shanti Swarup Bhatnagar Prize for the years 2016, Bhawan, New Delhi, on 28 February 2019. CSIR News February 2019 21 and technology-based In The News industries, Mr Modi asked the scientific community to take advantage of the Fourth Industrial Revolution and develop technologies. Highlighting the need to think beyond silos, the Prime Minister further added that scientists and researchers should The Hon’ble PM adopt an interdisciplinary Shri Narendra Modi approach which would said that science, help in finding faster and better solutions for technology and the various scientific innovation should questions. be connected with Honourable PM Shri Narendra Modi called on scientists and During his address researchers to adopt an interdisciplinary approach the aspirations and at the award ceremony, Dr Harsh Vardhan, requirements of the The Honourable PM Shri Narendra Union Science & Technology Minister, society. Modi congratulated and appreciated said that the efforts of the scientific the awardees and said that science, community have taken CSIR to the technology and innovation should be ninth position in international ranking connected with the aspirations and out of 1207 institutions of the world. requirements of the society. He also Further, he mentioned about the added that our scientific institutions various Government initiatives to foster should align with future requirements innovation and strengthen the Science and try to find solutions for local and Technology space in India.