November 23, 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

Calendar September 14, 2020 9:30 AM Commissioners Session
Calendar September 14, 2020 9:30 AM Commissioners Session September 17, 2020 9:30 AM Commissioners Session 10:00 AM Reconvening Of Public Hearing For Consideration Of Pither #377 Drainage Maintenance Petition (Public Participation In This Hearing Will Be Taking Place Only By Virtual Means) 1:30 PM Work Session September 21, 2020 9:30 AM Commissioners Session September 24, 2020 Canceled -NO Session September 28, 2020 9:30 AM Commissioners Session 1:30 PM View, With The Use Of Video Technology, For The Petition Requesting The Vacation Of A 0.165 Acre Tract Of Bainbridge Mills Drive In Liberty Township (Public Participation In This Viewing, Will Be Taking Place Only By Virtual Means) October 1, 2020 9:30 AM Commissioners Session 10:00 AM Reconvening Of Hearing For Consideration Of The Zerbe- O’Keefe #265 Drainage Improvement Petition October 5, 2020 9:30 AM Commissioners Session 10:00 AM Hearing For The Petition Requesting The Vacation Of A 0.165 Acre Tract Of Bainbridge Mills Drive In Liberty Township (Public Participation In This Viewing, Will Be Taking Place Only By Virtual Means) October 8, 2020 9:30 AM Commissioners Session October 12, 2020 9:30 AM Commissioners Session October 15, 2020 9:30 AM Commissioners Session October 19, 2020 9:30 AM Commissioners Session October 22, 2020 9:30 AM Commissioners Session October 22, 2020 10:00 AM Reconvene Public Hearing For Consideration Of A Petition From The Lake-Of-The-Woods Water Company Requesting Dedication Of A 3.136-Acre Segment Of Duncan’s Glen Drive As A Public Right-Of-Way October 26, -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

20-21 MHS FALL CALENDAR.Pdf
Monterey High School Monterey High School st nd Fall 1 Quarter: 8/17/20-10/16/20 Fall 2 Quarter: 10/19/20 – 12/18/20 SUNDAY MONDAY TUESDAY WEDNESDAY THURSDAY FRIDAY SATURDAY SUNDAY MONDAY TUESDAY WEDNESDAY THURSDAY FRIDIAY SATURDAY August 2 August 3 August 4 August 5 August 6 August 7 August 8 August 9 August 10 August 11 August 12 August 13 August 14 August 15 District Staff District Staff District Staff Teacher Teacher Development Development Development Work Day Work Day August 16 August 17 August 18 August 19 August 20 August 21 August 22 October 18 October 19 October 20 October 21 October 22 October 23 October 24 Instruction Start New Qtr Begins August 23 August 24 August 25 August 26 August 27 August 28 August 29 October 25 October 26 October 27 October 28 October 29 October 30 October 31 August 30 August 31 September 1 September 2 September 3 September 4 Sept 5 November 1 November 2 November 3 November 4 November 5 November 6 November 7 Sept 6 September 7 September 8 September 9 September September 11 Sept 12 November 8 November 9 November 10 November 11 November November 13 November 14 10 12 Labor Day Back to Veteran’s School Night Day Sept 13 September 14 September 15 September 16 September September 18 Sept 19 November 15 November 16 November 17 November 18 November November 20 November 21 17 19 September 21 September 22 September 23 September September 25 Sept 26 November 22 November 23 November 24 November 25 November November 27 November 28 24 26 NO SCHOOL Thanksgiving No School Sept 27 September 28 September 29 September -
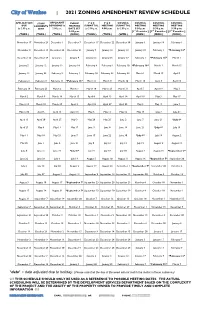
2021 Rezoning Review Schedule
City of Waukee | 2021 ZONING AMENDMENT REVIEW SCHEDULE APPLICATION STAFF APPLICANT PUBLIC P & Z P & Z COUNCIL COUNCIL COUNCIL COUNCIL DUE COMMENTS REVISIONS by HEARING SUBMITTAL MEETING SUBMITTAL MEETING MEETING MEETING by 5:00 p.m. SENT 5:00 p.m. DATE SET by 5:00 p.m. 6:00 p.m. by 5:00 p.m. 5:30 p.m. 5:30 p.m. 5:30 p.m. 5:30 p.m. [1st Consider.] [2nd Consider.] [3rd Consider.] (TUES.) (TUES.) (TUES.) (MON.) (THUR.) (TUES.) (WED.) (MON.) (MON.) (MON.) November 17 November 24 December 1 December 7 December 17 December 22 December 30 January 4 January 18 February 1 December 8 December 15 December 22 December 21 January 7 January 12 January 13 January 18 February 1 *February 16* December 22 December 29 January 5 January 4 January 21 January 26 January 27 February 1 *February 16* March 1 January 5 January 12 January 19 January 18 February 4 February 9 February 10 *February 16* March 1 March 15 January 19 January 26 February 2 February 1 February 18 February 23 February 24 March 1 March 15 April 5 February 2 February 9 February 16 *February 16* March 4 March 9 March 10 March 15 April 5 April 19 February 16 February 23 March 2 March 1 March 18 March 23 March 31 April 5 April 19 May 3 March 2 March 9 March 16 March 15 April 8 April 13 April 14 April 19 May 3 May 17 March 16 March 23 March 30 April 5 April 22 April 27 April 28 May 3 May 17 June 7 March 30 April 6 April 13 April 19 May 6 May 11 May 12 May 17 June 7 June 21 April 13 April 20 April 27 May 3 May 20 May 25 June 2 June 7 June 21 *July 6* April 27 May 4 May 11 May 17 June 3 June 8 June -

Meeting Deadlines
2021 PLANNING COMMISSION DEADLINE DATES Meeting Date Submit Pre-ap Plan Pre-ap Meeting Deadline Application Deadline 10 am Meeting Date January 6, 2021 November 23, 2021 November 25, 2021 December 3, 2020 January 6, 2021 January 20, 2021 December 8, 2020 December 10, 2020 December 17, 2020 January 20, 2021 February 3, 2021 December 14, 2020 December 16, 2020 December 23, 2020 February 3, 2021 February 17, 2021 January 5, 2021 January 7, 2021 January 14, 2021 February 17, 2021 March 3, 2021 January 19, 2021 January 21, 2021 January 28, 2021 March 3, 2021 March 17, 2021 February 2, 2021 February 4, 2021 February 11, 2021 March 17, 2021 April 7, 2021 February 23, 2021 February 25, 2021 March 4, 2021 April 7, 2021 April 21, 2021 March 9, 2021 March 11, 2021 March 18, 2021 April 21, 2021 May 5, 2021 March 23, 2021 March 25, 2021 April 1, 2021 May 5, 2021 May 19, 2021 April 6, 2021 April 8, 2021 April 15, 2021 May 19, 2021 June 2, 2021 April 20, 2021 April 22, 2021 April 29, 2021 June 2, 2021 June 16, 2021 May 4, 2021 May 6, 2021 May 13, 2021 June 16, 2021 July 7, 2021 May 25, 2021 May 27, 2021 June 3, 2021 July 7, 2021 July 21, 2021 June 8, 2021 June 10, 2021 June 17, 2021 July 21, 2021 August 4, 2021 June 22, 2021 June 24, 2021 July 1, 2021 August 4, 2021 August 18, 2021 July 6, 2021 July 8, 2021 July 15, 2021 August 18, 2021 September 1, 2021 July 20, 2021 July 22, 2021 July 29, 2021 September 1, 2021 September 15, 2021 August 3, 2021 August 5, 2021 August 12, 2021 September 15, 2021 October 6, 2021 August 24, 2021 August 26, 2021 September -

State of Illinois COVID-19 Update Nov
COVID-19 Update State of Illinois Monday, November 23, 2020 PANDEMIC-RELATED UNEMPLOYMENT EXCEEDS PREVIOUS RECESSIONS Cumulative Regular UI Initial Claims Comparison to Recent Recessions 2,400,000 2,000,000 1,600,000 1,200,000 800,000 400,000 0 1 5 9 13 17 21 25 29 33 37 41 45 49 53 57 61 65 69 73 77 81 Weeks Since Beginning of Recession 2001 Recession 2008 Recession 2020 Recession State of Illinois COVID-19 Update Nov. 23, 2020 ILLINOIS RESPONDS TO SUPPORT WORKERS IDES efforts to increase access to benefits and support employers Easing Certification Requirements Waiving the Waiting Week • Assisting individuals laid off due to a temporary closing • With support from Governor and legislature, waived UI of a business to continue to qualify for benefits. waiting week, granting claimants an extra week of • Expanding the definition of “able and available to work” benefits during their first payment. to help claimants certify. Non-Instructional Academic Statements of Benefit Charges Employees • With legislature’s support, adjusted employer reporting • With legislature’s support, accommodated a change in system to ensure employer tax rates and reimbursable eligibility for non-instructional academic employees. wouldn’t rise due to pandemic-induced claims. State of Illinois COVID-19 Update Nov. 23, 2020 SUPPORTING CLAIMANTS IN RECORD NUMBERS IDES has paid out over 16x more benefits compared to 2019, assisting 1.3 million people statewide. Benefits Paid (March through October) Program 2019 2020 Regular UI $1.056 billion $5.422 billion FPUC $0 $8.914 billion PEUC $0 $514 million PUA $0 $1.574 billion EB (CARES) $0 $109 million LWA $0 $1.127 billion Total Benefits Paid $1.056 billion $17.659 billion State of Illinois COVID-19 Update Nov. -

Utility Billing Dates 2021
2021 Reading & Billing Schedule for Website.xlsx Billing Month Cycle Start Read date Finish read date Billing Date Due Date & Bank Draft Date Penalty Date Pay by dates to avoid cut off Cut off date January 1 Monday, December 21, 2020 Thursday, December 24, 2020 Thursday, January 7, 2021 Thursday, January 28, 2021 Tuesday, February 2, 2021 Tuesday, February 16, 2021 Wednesday, February 17, 2021 2 Monday, December 28, 2020 Thursday, December 31, 2020 Thursday, January 14, 2021 Thursday, February 4, 2021 Tuesday, February 9, 2021 Tuesday, February 23, 2021 Wednesday, February 24, 2021 3 Monday, January 4, 2021 Thursday, January 7, 2021 Thursday, January 21, 2021 Thursday, February 11, 2021 Tuesday, February 16, 2021 Tuesday, March 2, 2021 Wednesday, March 3, 2021 4 Monday, January 11, 2021 Thursday, January 14, 2021 Thursday, January 28, 2021 Thursday, February 18, 2021 Tuesday, February 23, 2021 Tuesday, March 9, 2021 Wednesday, March 10, 2021 February 1 Tuesday, January 19, 2021 Friday, January 22, 2021 Thursday, February 4, 2021 Thursday, February 25, 2021 Tuesday, March 2, 2021 Tuesday, March 16, 2021 Wednesday, March 17, 2021 2 Monday, January 25, 2021 Thursday, January 28, 2021 Thursday, February 11, 2021 Thursday, March 4, 2021 Tuesday, March 9, 2021 Tuesday, March 23, 2021 Wednesday, March 24, 2021 3 Monday, February 1, 2021 Thursday, February 4, 2021 Thursday, February 18, 2021 Thursday, March 11, 2021 Tuesday, March 16, 2021 Tuesday, March 30, 2021 Wednesday, March 31, 2021 4 Monday, February 8, 2021 Thursday, February 11, 2021 -

November 23, 2020
TCA Newsletter November 23, 2020 Upcoming Important Dates SCHEDULE CHANGES - THANKSGIVING WEEK During the week of November 23rd (Thanksgiving Week) November 26 & 27 - No school, Thanksgiving December 8 - Virtual Info Session A-Day Monday (periods 1-4) December 23 - No School, Winter Break Begins B-Day Tuesday (periods 5-8) January 4 - School Resumes C-Day Wednesday (100% Asynchronous) No School Thursday and Friday Lueth Letter Dear TCA families, Wednesday Schedule Change As we have finished up quarter one, we are Beginning on Wednesday December 2, 2020 looking forward to quarter two and beyond. Per the Governor’s Executive Order 20-94 all Minnesota To make sure that we are serving your public schools who are serving students in either a distance family and student(s) in the best possible way, we are learning or hybrid model are required to allow an additional seeking your input through our short family survey. You can 150 minutes per week for teacher preparation time. find the link to that survey in this newsletter, below. This new Executive Order combined with the need for more We are also looking forward to meeting with all of you student support time, assignment work time, and to help tomorrow, Saturday November 21st, during conferences. with Zoom fatigue, has led TCA to adopt a new plan for C Conferences are a great time to have individual Days (Wednesdays) for the remainder of the school year conversations with your student's advisor to share how regardless of what Phase we are in. things are going as well. Beginning on Wednesday December 2, 2020, following will It seems that, unfortunately due to the growing cases of be the new schedule for students and teachers.