A Practical Guide to Selecting the Right Control Chart
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
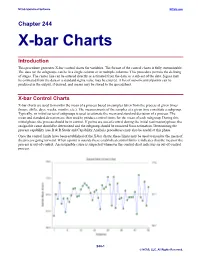
X-Bar Charts
NCSS Statistical Software NCSS.com Chapter 244 X-bar Charts Introduction This procedure generates X-bar control charts for variables. The format of the control charts is fully customizable. The data for the subgroups can be in a single column or in multiple columns. This procedure permits the defining of stages. The center line can be entered directly or estimated from the data, or a sub-set of the data. Sigma may be estimated from the data or a standard sigma value may be entered. A list of out-of-control points can be produced in the output, if desired, and means may be stored to the spreadsheet. X-bar Control Charts X-bar charts are used to monitor the mean of a process based on samples taken from the process at given times (hours, shifts, days, weeks, months, etc.). The measurements of the samples at a given time constitute a subgroup. Typically, an initial series of subgroups is used to estimate the mean and standard deviation of a process. The mean and standard deviation are then used to produce control limits for the mean of each subgroup. During this initial phase, the process should be in control. If points are out-of-control during the initial (estimation) phase, the assignable cause should be determined and the subgroup should be removed from estimation. Determining the process capability (see R & R Study and Capability Analysis procedures) may also be useful at this phase. Once the control limits have been established of the X-bar charts, these limits may be used to monitor the mean of the process going forward. -
D Quality and Reliability Engineering 4 0 0 4 4 3 70 30 20 0
B.E Semester: VII Mechanical Engineering Subject Name: Quality and Reliability Engineering A. Course Objective To present a problem oriented in depth knowledge of Quality and Reliability Engineering. To address the underlying concepts, methods and application of Quality and Reliability Engineering. B. Teaching / Examination Scheme Teaching Scheme Total Evaluation Scheme Total SUBJECT Credit PR. / L T P Total THEORY IE CIA VIVO Marks CODE NAME Hrs Hrs Hrs Hrs Hrs Marks Marks Marks Marks Quality and ME706- Reliability 4 0 0 4 4 3 70 30 20 0 120 D Engineering C. Detailed Syllabus 1. Introduction: Quality – Concept, Different Definitions and Dimensions, Inspection, Quality Control, Quality Assurance and Quality Management, Quality as Wining Strategy, Views of different Quality Gurus. 2. Total Quality Management TQM: Introduction, Definitions and Principles of Operation, Tools and Techniques, such as, Quality Circles, 5 S Practice, Total Quality Control (TQC), Total Employee Involvement (TEI), Problem Solving Process, Quality Function Deployment (QFD), Failure Mode and Effect analysis (FMEA), Fault Tree Analysis (FTA), Kizen, Poka-Yoke, QC Tools, PDCA Cycle, Quality Improvement Tools, TQM Implementation and Limitations. 3. Introduction to Design of Experiments: Introduction, Methods, Taguchi approach, Achieving robust design, Steps in experimental design 4. Just –in –Time and Quality Management: Introduction to JIT production system, KANBAN system, JIT and Quality Production. 5. Introduction to Total Productive Maintenance (TPM): Introduction, Content, Methods and Advantages 6. Introduction to ISO 9000, ISO 14000 and QS 9000: Basic Concepts, Scope, Implementation, Benefits, Implantation Barriers 7. Contemporary Trends: Concurrent Engineering, Lean Manufacturing, Agile Manufacturing, World Class Manufacturing, Cost of Quality (COQ) system, Bench Marking, Business Process Re-engineering, Six Sigma - Basic Concept, Principle, Methodology, Implementation, Scope, Advantages and Limitation of all as applicable. -

Quality Assurance: Best Practices in Clinical SAS® Programming
NESUG 2012 Management, Administration and Support Quality Assurance: Best Practices in Clinical SAS® Programming Parag Shiralkar eClinical Solutions, a Division of Eliassen Group Abstract SAS® programmers working on clinical reporting projects are often under constant pressure of meeting tight timelines, producing best quality SAS® code and of meeting needs of customers. As per regulatory guidelines, a typical clinical report or dataset generation using SAS® software is considered as software development. Moreover, since statistical reporting and clinical programming is a part of clinical trial processes, such processes are required to follow strict quality assurance guidelines. While SAS® programmers completely focus on getting best quality deliverable out in a timely manner, quality assurance needs often get lesser priorities or may get unclearly understood by clinical SAS® programming staff. Most of the quality assurance practices are often focused on ‘process adherence’. Statistical programmers using SAS® software and working on clinical reporting tasks need to maintain adequate documentation for the processes they follow. Quality control strategy should be planned prevalently before starting any programming work. Adherence to standard operating procedures, maintenance of necessary audit trails, and necessary and sufficient documentation are key aspects of quality. This paper elaborates on best quality assurance practices which statistical programmers working in pharmaceutical industry are recommended to follow. These quality practices -

Data Quality Monitoring and Surveillance System Evaluation
TECHNICAL DOCUMENT Data quality monitoring and surveillance system evaluation A handbook of methods and applications www.ecdc.europa.eu ECDC TECHNICAL DOCUMENT Data quality monitoring and surveillance system evaluation A handbook of methods and applications This publication of the European Centre for Disease Prevention and Control (ECDC) was coordinated by Isabelle Devaux (senior expert, Epidemiological Methods, ECDC). Contributing authors John Brazil (Health Protection Surveillance Centre, Ireland; Section 2.4), Bruno Ciancio (ECDC; Chapter 1, Section 2.1), Isabelle Devaux (ECDC; Chapter 1, Sections 3.1 and 3.2), James Freed (Public Health England, United Kingdom; Sections 2.1 and 3.2), Magid Herida (Institut for Public Health Surveillance, France; Section 3.8 ), Jana Kerlik (Public Health Authority of the Slovak Republic; Section 2.1), Scott McNabb (Emory University, United States of America; Sections 2.1 and 3.8), Kassiani Mellou (Hellenic Centre for Disease Control and Prevention, Greece; Sections 2.2, 2.3, 3.3, 3.4 and 3.5), Gerardo Priotto (World Health Organization; Section 3.6), Simone van der Plas (National Institute of Public Health and the Environment, the Netherlands; Chapter 4), Bart van der Zanden (Public Health Agency of Sweden; Chapter 4), Edward Valesco (Robert Koch Institute, Germany; Sections 3.1 and 3.2). Project working group members: Maria Avdicova (Public Health Authority of the Slovak Republic), Sandro Bonfigli (National Institute of Health, Italy), Mike Catchpole (Public Health England, United Kingdom), Agnes -

Quality Control and Reliability Engineering 1152Au128 3 0 0 3
L T P C QUALITY CONTROL AND RELIABILITY ENGINEERING 1152AU128 3 0 0 3 1. Preamble This course provides the essentiality of SQC, sampling and reliability engineering. Study on various types of control charts, six sigma and process capability to help the students understand various quality control techniques. Reliability engineering focuses on the dependability, failure mode analysis, reliability prediction and management of a system 2. Pre-requisite NIL 3. Course Outcomes Upon the successful completion of the course, learners will be able to Level of learning CO Course Outcomes domain (Based on Nos. C01 Explain the basic concepts in Statistical Process Control K2 Apply statistical sampling to determine whether to accept or C02 K2 reject a production lot Predict lifecycle management of a product by applying C03 K2 reliability engineering techniques. C04 Analyze data to determine the cause of a failure K2 Estimate the reliability of a component by applying RDB, C05 K2 FMEA and Fault tree analysis. 4. Correlation with Programme Outcomes Cos PO1 PO2 PO3 PO4 PO5 PO6 PO7 PO8 PO9 PO10 PO11 PO12 PSO1 PSO2 CO1 H H H M L H M L CO2 H H H M L H H M CO3 H H H M L H L H CO4 H H H M L H M M CO5 H H H M L H L H H- High; M-Medium; L-Low 5. Course content UNIT I STATISTICAL QUALITY CONTROL L-9 Methods and Philosophy of Statistical Process Control - Control Charts for Variables and Attributes Cumulative Sum and Exponentially Weighted Moving Average Control Charts - Other SPC Techniques Process - Capability Analysis - Six Sigma Concept. -

A Guide to Creating and Interpreting Run and Control Charts Turning Data Into Information for Improvement Using This Guide
Institute for Innovation and Improvement A guide to creating and interpreting run and control charts Turning Data into Information for Improvement Using this guide The NHS Institute has developed this guide as a reminder to commissioners how to create and analyse time-series data as part of their improvement work. It should help you ask the right questions and to better assess whether a change has led to an improvement. Contents The importance of time based measurements .........................................4 Run Charts ...............................................6 Control Charts .......................................12 Process Changes ....................................26 Recommended Reading .........................29 The Improving immunisation rates importance Before and after the implementation of a new recall system This example shows yearly figures for immunisation rates of time-based before and after a new system was introduced. The aggregated measurements data seems to indicate the change was a success. 90 Wow! A “significant improvement” from 86% 79% to 86% -up % take 79% 75 Time 1 Time 2 Conclusion - The change was a success! 4 Improving immunisation rates Before and after the implementation of a new recall system However, viewing how the rates have changed within the two periods tells a 100 very different story. Here New system implemented here we see that immunisation rates were actually improving until the new 86% – system was introduced. X They then became worse. 79% – Seeing this more detailed X 75 time based picture prompts a different response. % take-up Now what do you conclude about the impact of the new system? 50 24 Months 5 Run Charts Elements of a run chart A run chart shows a measurement on the y-axis plotted over time (on the x-axis). -

Statistical Quality Control Methods in Infection Control and Hospital Epidemiology, Part I: Introduction and Basic Theory Author(S): James C
Statistical Quality Control Methods in Infection Control and Hospital Epidemiology, Part I: Introduction and Basic Theory Author(s): James C. Benneyan Source: Infection Control and Hospital Epidemiology, Vol. 19, No. 3 (Mar., 1998), pp. 194-214 Published by: The University of Chicago Press Stable URL: http://www.jstor.org/stable/30143442 Accessed: 25/06/2010 18:26 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=ucpress. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. The University of Chicago Press is collaborating with JSTOR to digitize, preserve and extend access to Infection Control and Hospital Epidemiology. -

Medians and the Individuals Control Chart the Individuals Control Chart
Medians and the Individuals Control Chart The individuals control chart is used quite often to monitor processes. It can be used in almost all cases, so it makes the art of deciding which control chart to use extremely easy at times. Not sure? Use the individuals control chart. The individuals control chart is composed of two charts: the X chart where the individual values are plotted and the moving range (mR) chart where the range between consecutive values are plotted. The averages and control limits are also part of the charts. The average moving range (R̅) is used to calculate the control limits on the X chart. If the mR chart has a lot of out of control points, the average moving range will be inflated leading to wider control limits on the X chart. This can lead to missed signals (out of control points) on the X chart. Is there anything you can do when the mR chart has many out of control points to help miss fewer signals on the X chart? Yes, there is. One method is to use the median moving range. The median moving range is impacted much less by large moving range values than the average. Another option is to use the median of the X values in place of the overall average on the X chart. This publication explores using median values for the centerlines on the X and mR charts. We will start with a review of the individuals control chart. Then, the individuals control charts using median values is introduced. The charts look the same – the only difference is the location of the centerlines and control limits. -

Methodological Challenges Associated with Meta-Analyses in Health Care and Behavioral Health Research
o title: Methodological Challenges Associated with Meta Analyses in Health Care and Behavioral Health Research o date: May 14, 2012 Methodologicalo author: Challenges Associated with Judith A. Shinogle, PhD, MSc. Meta-AnalysesSenior Research Scientist in Health , Maryland InstituteCare for and Policy Analysis Behavioraland Research Health Research University of Maryland, Baltimore County 1000 Hilltop Circle Baltimore, Maryland 21250 http://www.umbc.edu/mipar/shinogle.php Judith A. Shinogle, PhD, MSc Senior Research Scientist Maryland Institute for Policy Analysis and Research University of Maryland, Baltimore County 1000 Hilltop Circle Baltimore, Maryland 21250 http://www.umbc.edu/mipar/shinogle.php Please see http://www.umbc.edu/mipar/shinogle.php for information about the Judith A. Shinogle Memorial Fund, Baltimore, MD, and the AKC Canine Health Foundation (www.akcchf.org), Raleigh, NC. Inquiries may also be directed to Sara Radcliffe, Executive Vice President for Health, Biotechnology Industry Organization, www.bio.org, 202-962-9200. May 14, 2012 Methodological Challenges Associated with Meta-Analyses in Health Care and Behavioral Health Research Judith A. Shinogle, PhD, MSc I. Executive Summary Meta-analysis is used to inform a wide array of questions ranging from pharmaceutical safety to the relative effectiveness of different medical interventions. Meta-analysis also can be used to generate new hypotheses and reflect on the nature and possible causes of heterogeneity between studies. The wide range of applications has led to an increase in use of meta-analysis. When skillfully conducted, meta-analysis is one way researchers can combine and evaluate different bodies of research to determine the answers to research questions. However, as use of meta-analysis grows, it is imperative that the proper methods are used in order to draw meaningful conclusions from these analyses. -

Phase I and Phase II - Control Charts for the Variance and Generalized Variance
Phase I and Phase II - Control Charts for the Variance and Generalized Variance R. van Zyl1, A.J. van der Merwe2 1Quintiles International, [email protected] 2University of the Free State 1 Abstract By extending the results of Human, Chakraborti, and Smit(2010), Phase I control charts are derived for the generalized variance when the mean vector and covariance matrix of multivariate normally distributed data are unknown and estimated from m independent samples, each of size n. In Phase II predictive distributions based on a Bayesian approach are used to construct Shewart-type control limits for the variance and generalized variance. The posterior distribution is obtained by combining the likelihood (the observed data in Phase I) and the uncertainty of the unknown parameters via the prior distribution. By using the posterior distribution the unconditional predictive density functions are derived. Keywords: Shewart-type Control Charts, Variance, Generalized Variance, Phase I, Phase II, Predictive Density 1 Introduction Quality control is a process which is used to maintain the standards of products produced or services delivered. It is nowadays commonly accepted by most statisticians that statistical processes should be implemented in two phases: 1. Phase I where the primary interest is to assess process stability; and 2. Phase II where online monitoring of the process is done. Bayarri and Garcia-Donato(2005) gave the following reasons for recommending Bayesian analysis for the determining of control chart limits: • Control charts are based on future observations and Bayesian methods are very natural for prediction. • Uncertainty in the estimation of the unknown parameters are adequately handled. -
Introduction to Using Control Charts Brought to You by NICHQ
Introduction to Using Control Charts Brought to you by NICHQ Control Chart Purpose A control chart is a statistical tool that can help users identify variation and use that knowledge to inform the development of changes for improvement. Control charts provide a method to distinguish between the two types of causes of variation in a measure: Common Causes - those causes that are inherent in the system over time, affect everyone working in the system, and affect all outcomes of the system. Using your morning commute as an example, it may take between 35-45 minutes to commute to work each morning. It does not take exactly 40 minutes each morning because there is variation in common causes, such as the number of red lights or traffic volume. Special Causes - those causes that are not part of the system all the time or do not affect everyone, but arise because of specific circumstances. For example, it may take you 2 hours to get to work one morning because of a special cause, such as a major accident. Variation in data is expected and the type of variation that affects your system will inform your course of action for making improvements. A stable system, or one that is in a state of statistical control, is a system that has only common causes affecting the outcomes (common cause variation only). A stable system implies that the variation is predictable within statistically established limits, but does not imply that the system is performing well. An unstable system is a system with both common and special causes affecting the outcomes. -

University Quality Indicators: a Critical Assessment
DIRECTORATE GENERAL FOR INTERNAL POLICIES POLICY DEPARTMENT B: STRUCTURAL AND COHESION POLICIES CULTURE AND EDUCATION UNIVERSITY QUALITY INDICATORS: A CRITICAL ASSESSMENT STUDY This document was requested by the European Parliament's Committee on Culture and Education AUTHORS Bernd Wächter (ACA) Maria Kelo (ENQA) Queenie K.H. Lam (ACA) Philipp Effertz (DAAD) Christoph Jost (DAAD) Stefanie Kottowski (DAAD) RESPONSIBLE ADMINISTRATOR Mr Miklós Györffi Policy Department B: Structural and Cohesion Policies European Parliament B-1047 Brussels E-mail: [email protected] EDITORIAL ASSISTANCE Lyna Pärt LINGUISTIC VERSIONS Original: EN ABOUT THE EDITOR To contact the Policy Department or to subscribe to its monthly newsletter please write to: [email protected] Manuscript completed in April 2015. © European Parliament, 2015. Print ISBN 978-92-823-7864-9 doi: 10.2861/660770 QA-02-15-583-EN-C PDF ISBN 978-92-823-7865-6 doi: 10.2861/426164 QA-02-15-583-EN-N This document is available on the Internet at: http://www.europarl.europa.eu/studies DISCLAIMER The opinions expressed in this document are the sole responsibility of the author and do not necessarily represent the official position of the European Parliament. Reproduction and translation for non-commercial purposes are authorized, provided the source is acknowledged and the publisher is given prior notice and sent a copy. DIRECTORATE GENERAL FOR INTERNAL POLICIES POLICY DEPARTMENT B: STRUCTURAL AND COHESION POLICIES CULTURE AND EDUCATION UNIVERSITY QUALITY INDICATORS: A CRITICAL ASSESSMENT STUDY Abstract The ‘Europe 2020 Strategy’ and other EU initiatives call for more excellence in Europe’s higher education institutions in order to improve their performance, international attractiveness and competitiveness.