Quality Assurance/Quality Control Officer Is a Fully-Experienced, Single-Position Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomic Modeling Using 3D Printing: Quality Assurance and Optimization
Leng et al. 3D Printing in Medicine (2017) 3:6 DOI 10.1186/s41205-017-0014-3 RESEARCH Open Access Anatomic modeling using 3D printing: quality assurance and optimization Shuai Leng1*, Kiaran McGee1, Jonathan Morris1, Amy Alexander1, Joel Kuhlmann2, Thomas Vrieze1, Cynthia H. McCollough1 and Jane Matsumoto1 Abstract Background: The purpose of this study is to provide a framework for the development of a quality assurance (QA) program for use in medical 3D printing applications. An interdisciplinary QA team was built with expertise from all aspects of 3D printing. A systematic QA approach was established to assess the accuracy and precision of each step during the 3D printing process, including: image data acquisition, segmentation and processing, and 3D printing and cleaning. Validation of printed models was performed by qualitative inspection and quantitative measurement. The latter wasachievedbyscanningtheprintedmodelwithahighresolution CT scanner to obtain images of the printed model, which were registered to the original patient images and the distance between them was calculated on a point-by-point basis. Results: A phantom-based QA process, with two QA phantoms, was also developed. The phantoms went through the same 3D printing process as that of the patient models to generate printed QA models. Physical measurement, fit tests, and image based measurements were performed to compare the printed 3D model to the original QA phantom, with its known size and shape, providing an end-to-end assessment of errors involved in the complete 3D printing process. Measured differences between the printed model and the original QA phantom ranged from -0.32 mm to 0.13 mm for the line pair pattern. -

Construction Quality Control/Quality Assurance Plan Bozeman Landfill LFG/SVE/AI and Treatment System City of Bozeman Landfill;
Construction Quality Control/Quality Assurance Plan Bozeman Landfill LFG/SVE/AI and Treatment System City of Bozeman Landfill; Bozeman, MT #114-560487 July 10, 2015 PRESENTED TO PRESENTED BY City of Bozeman Tetra Tech, Inc. P +1-406-443-5210 PO Box 1230 303 Irene Street F +1-406-449-3729 Bozeman, MT 59711-1230 Helena, MT 59601 tetratech.com Prepared by: Mary Bell July 10, 2015 Reviewed by: Larry Cawlfield, P.E., P.H. July 10, 2015 Engineer/Hydrologist Authorized by: Larry Cawlfield, P.E., P.H. July 10, 2015 Engineer/Hydrologist Construction Quality Control/Quality Assurance Plan LFG/SVE/AI and Treatment System TABLE OF CONTENTS 1.0 INTRODUCTION ..................................................................................................................................................1 1.1 CQCQAP Organization ..................................................................................................................................1 2.0 PROJECT QA/QC ORGANIZATION ...................................................................................................................2 2.1 Responsibilities and Authorities of Key Personnel ........................................................................................2 2.1.1 Engineer of Record ...............................................................................................................................2 2.1.2 QA On-Site Supervisor .........................................................................................................................3 2.1.3 QC -

Examples of Quality Control and Quality Assurance During Construction
Examples of Quality Control and Quality Assurance During Construction Prepared by the Construction Practices Subcommittee Of APWA’s UPROW Committee Introduction The UPROW Committee requested that the Construction Practices Subcommittee research and evaluate the existing available documents related to Quality Assurance and Quality Control (QA/QC) during construction. The request initiated from the report Recommendations to Establish a New Professional / Educational / Technical Committee for Utility and Public Right-of-Way Issues, prepared by the Utility and Right-of-Way Task Force, and dated April 13, 1998. The following report documents the process that the subcommittee followed in gathering, assessing, evaluating and reporting the data and the results. Data Collection The Subcommittee chair requested that each member search for examples of specifications, guidelines, manuals, programs or other written information, which outlined methods or requirements of QA/QC during construction. The members of the subcommittee were located in various regions across the entire country, from governmental (local, state and federal), industrial, commercial and private consulting backgrounds. A total of nine documents were received and reviewed. While the number of documents was not expansive, it was the opinion of the subcommittee that the sources of these documents have extensive background in construction quality control and therefore these documents cover the topic adequately. It is likely that other agencies have very good examples of standards but the subcommittee was unable to obtain other documents in their search. Analysis and Evaluation Prior to review the documents, members of the subcommittee developed a list of criteria to allow a subjective evaluation of the sample documents. These criteria included the following factors: · Quality Control Organization Items considered: The classifications of the employees included within the structure of the organization. -
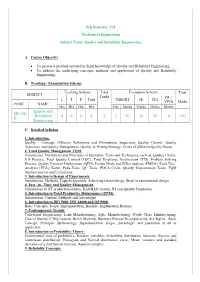
D Quality and Reliability Engineering 4 0 0 4 4 3 70 30 20 0
B.E Semester: VII Mechanical Engineering Subject Name: Quality and Reliability Engineering A. Course Objective To present a problem oriented in depth knowledge of Quality and Reliability Engineering. To address the underlying concepts, methods and application of Quality and Reliability Engineering. B. Teaching / Examination Scheme Teaching Scheme Total Evaluation Scheme Total SUBJECT Credit PR. / L T P Total THEORY IE CIA VIVO Marks CODE NAME Hrs Hrs Hrs Hrs Hrs Marks Marks Marks Marks Quality and ME706- Reliability 4 0 0 4 4 3 70 30 20 0 120 D Engineering C. Detailed Syllabus 1. Introduction: Quality – Concept, Different Definitions and Dimensions, Inspection, Quality Control, Quality Assurance and Quality Management, Quality as Wining Strategy, Views of different Quality Gurus. 2. Total Quality Management TQM: Introduction, Definitions and Principles of Operation, Tools and Techniques, such as, Quality Circles, 5 S Practice, Total Quality Control (TQC), Total Employee Involvement (TEI), Problem Solving Process, Quality Function Deployment (QFD), Failure Mode and Effect analysis (FMEA), Fault Tree Analysis (FTA), Kizen, Poka-Yoke, QC Tools, PDCA Cycle, Quality Improvement Tools, TQM Implementation and Limitations. 3. Introduction to Design of Experiments: Introduction, Methods, Taguchi approach, Achieving robust design, Steps in experimental design 4. Just –in –Time and Quality Management: Introduction to JIT production system, KANBAN system, JIT and Quality Production. 5. Introduction to Total Productive Maintenance (TPM): Introduction, Content, Methods and Advantages 6. Introduction to ISO 9000, ISO 14000 and QS 9000: Basic Concepts, Scope, Implementation, Benefits, Implantation Barriers 7. Contemporary Trends: Concurrent Engineering, Lean Manufacturing, Agile Manufacturing, World Class Manufacturing, Cost of Quality (COQ) system, Bench Marking, Business Process Re-engineering, Six Sigma - Basic Concept, Principle, Methodology, Implementation, Scope, Advantages and Limitation of all as applicable. -

Quality Assurance: Best Practices in Clinical SAS® Programming
NESUG 2012 Management, Administration and Support Quality Assurance: Best Practices in Clinical SAS® Programming Parag Shiralkar eClinical Solutions, a Division of Eliassen Group Abstract SAS® programmers working on clinical reporting projects are often under constant pressure of meeting tight timelines, producing best quality SAS® code and of meeting needs of customers. As per regulatory guidelines, a typical clinical report or dataset generation using SAS® software is considered as software development. Moreover, since statistical reporting and clinical programming is a part of clinical trial processes, such processes are required to follow strict quality assurance guidelines. While SAS® programmers completely focus on getting best quality deliverable out in a timely manner, quality assurance needs often get lesser priorities or may get unclearly understood by clinical SAS® programming staff. Most of the quality assurance practices are often focused on ‘process adherence’. Statistical programmers using SAS® software and working on clinical reporting tasks need to maintain adequate documentation for the processes they follow. Quality control strategy should be planned prevalently before starting any programming work. Adherence to standard operating procedures, maintenance of necessary audit trails, and necessary and sufficient documentation are key aspects of quality. This paper elaborates on best quality assurance practices which statistical programmers working in pharmaceutical industry are recommended to follow. These quality practices -

Construction Quality Assurance Plan
Assets I Engineering I Environment I Noise I Spatial I Waste Construction Quality Assurance Plan Pilbara Regional Waste Management Facility- Cell 1 Development and Associated Works shire of Ashburton reef to range Prepared for Shire of Ashburton February 2019 Project Number: TW17053 Construction Quality Assurance Plan Pi Ibara Regional Waste Management Facility - Cell 1 Development and Associated Works Shire of Ashburton talis delivering solutions DOCUMENT CONTROL 1 Version Description Date Author Reviewer J L ------------ ------------------ -- Oa Internal Review 15/01/19 cs LM la Released to Client 11/02/19 LM LM+EP Approval for Release Copyright of this document or any part of this document remains with Tatis Consultants Pty Ltd and cannot be used, transferred or reproduced in any manner or form without prior written consent from Tatis Consultants Pty Ltd. 1W17053 - CQA Plan.la February 2019 I Page i Construction Quality Assurance Plan Pi Ibara Regional Waste Management Facility - Cell 1 Development and Associated Works Shire of Ashburton talis Table of Contents 1 Introduction ......................................................................................................................... 1 2 Definitions ........................................................................................................................... 2 2.1 Material Definitions ................................................................................................................ 2 3 Roles of Participants ............................................................................................................ -

GLP 1 Quality Assurance of Laboratory Measurement Results
GLP 1 Good Laboratory Practice for the Quality Assurance of Laboratory Measurement Results1 Quality assurance of laboratory measurement results (measurement assurance) means understanding, modeling, measuring, and managing a measurement assurance system appropriate This publication is available free of charge from: https://doi.org/10.6028/NIST.IR.6969 to the laboratory’s scope of activities. Having such a system in place will allow the laboratory to know, within the limits of a measurement process, that measurement results are valid with respect to stated traceability, accuracy, and precision. A well-designed measurement assurance system provides confidence and credibility in the quality of the laboratory’s measurement results by ensuring that the measurement results are metrologically traceable to appropriate reference standards and measurement units, with suitably valid uncertainties. Quality assurance (QA) and quality control (QC) methods should consider both internal and external data for evaluating the ongoing stability and control of the measurement results and processes. At its core, the concept of measurement assurance is one of risk identification and mitigation. It provides methods for monitoring the standards and as well as the measurement process, and varying combinations of each depending on the priorities of the methods chosen. Software quality assurance is a key function of ensuring the quality of laboratory measurement results but is outside the scope of this procedure. 1 Internally Obtained Measurement Assurance Data The validity of calibrations needs to be monitored with quality control procedures. Statistical techniques are used to record, analyze, and monitor charted measurement results to permit the ongoing assurance of valid and stable measurement results, integration of intermediate checks, and/or the detection of trends. -

Data Quality Monitoring and Surveillance System Evaluation
TECHNICAL DOCUMENT Data quality monitoring and surveillance system evaluation A handbook of methods and applications www.ecdc.europa.eu ECDC TECHNICAL DOCUMENT Data quality monitoring and surveillance system evaluation A handbook of methods and applications This publication of the European Centre for Disease Prevention and Control (ECDC) was coordinated by Isabelle Devaux (senior expert, Epidemiological Methods, ECDC). Contributing authors John Brazil (Health Protection Surveillance Centre, Ireland; Section 2.4), Bruno Ciancio (ECDC; Chapter 1, Section 2.1), Isabelle Devaux (ECDC; Chapter 1, Sections 3.1 and 3.2), James Freed (Public Health England, United Kingdom; Sections 2.1 and 3.2), Magid Herida (Institut for Public Health Surveillance, France; Section 3.8 ), Jana Kerlik (Public Health Authority of the Slovak Republic; Section 2.1), Scott McNabb (Emory University, United States of America; Sections 2.1 and 3.8), Kassiani Mellou (Hellenic Centre for Disease Control and Prevention, Greece; Sections 2.2, 2.3, 3.3, 3.4 and 3.5), Gerardo Priotto (World Health Organization; Section 3.6), Simone van der Plas (National Institute of Public Health and the Environment, the Netherlands; Chapter 4), Bart van der Zanden (Public Health Agency of Sweden; Chapter 4), Edward Valesco (Robert Koch Institute, Germany; Sections 3.1 and 3.2). Project working group members: Maria Avdicova (Public Health Authority of the Slovak Republic), Sandro Bonfigli (National Institute of Health, Italy), Mike Catchpole (Public Health England, United Kingdom), Agnes -

Quality Control and Reliability Engineering 1152Au128 3 0 0 3
L T P C QUALITY CONTROL AND RELIABILITY ENGINEERING 1152AU128 3 0 0 3 1. Preamble This course provides the essentiality of SQC, sampling and reliability engineering. Study on various types of control charts, six sigma and process capability to help the students understand various quality control techniques. Reliability engineering focuses on the dependability, failure mode analysis, reliability prediction and management of a system 2. Pre-requisite NIL 3. Course Outcomes Upon the successful completion of the course, learners will be able to Level of learning CO Course Outcomes domain (Based on Nos. C01 Explain the basic concepts in Statistical Process Control K2 Apply statistical sampling to determine whether to accept or C02 K2 reject a production lot Predict lifecycle management of a product by applying C03 K2 reliability engineering techniques. C04 Analyze data to determine the cause of a failure K2 Estimate the reliability of a component by applying RDB, C05 K2 FMEA and Fault tree analysis. 4. Correlation with Programme Outcomes Cos PO1 PO2 PO3 PO4 PO5 PO6 PO7 PO8 PO9 PO10 PO11 PO12 PSO1 PSO2 CO1 H H H M L H M L CO2 H H H M L H H M CO3 H H H M L H L H CO4 H H H M L H M M CO5 H H H M L H L H H- High; M-Medium; L-Low 5. Course content UNIT I STATISTICAL QUALITY CONTROL L-9 Methods and Philosophy of Statistical Process Control - Control Charts for Variables and Attributes Cumulative Sum and Exponentially Weighted Moving Average Control Charts - Other SPC Techniques Process - Capability Analysis - Six Sigma Concept. -

Statistical Process Control and CAQ Systems As a Tools Assuring Quality
Statistical process control and CAQ systems as a tools assuring quality in the automotive industry doi:10.2478/mape-2019-0033 Date of submission to the Editor: 04/2018 MAPE 2019, volume 2, issue 1, pp. 336-344 Date of acceptance by the Editor: 07/2018 Łukasz Wiecha * MQA Łukasz Wiecha, Poland Grzegorz Ćwikła Silesian University of Technology, Poland INTRODUCTION At present, a market trend can be observed for the emergence of new enterprises characterised by a very large volume of production of a narrow range of products, using modern, high-performance technologies. With a large scale of production, it is not possible to completely eliminate products that do not meet the high quality criteria, however, it is necessary to reduce the number and probability of their occurrence, and to detect them at an early stage of the production process, which can be achieved by using appropriate statistical methods of process control based on the analysis of collected data (Kempa, 2011). Statistical methods of process control are based on Crosby's zero defects principle, which claimed that reliability and high production efficiency can only be achieved by eliminating errors and defects at all stages of the process. The main assumption of this principle is to eliminate the causes of non- compliance, not just their effects. For this reason, the main focus should be on ensuring quality at the source, by the earliest possible detection and elimination of a defect or problem. The author of this principle assumed that as a result of detecting nonconformities in the next stage of the process, the cost of this defect increases tenfold in relation to the previous operation, and determined that this goal can be achieved by empirically measuring the level of process quality and presenting the results in an easy to interpret form, which allows for effective corrective actions in a short time (Hamrol, 2005). -

Statistical Quality Control Methods in Infection Control and Hospital Epidemiology, Part I: Introduction and Basic Theory Author(S): James C
Statistical Quality Control Methods in Infection Control and Hospital Epidemiology, Part I: Introduction and Basic Theory Author(s): James C. Benneyan Source: Infection Control and Hospital Epidemiology, Vol. 19, No. 3 (Mar., 1998), pp. 194-214 Published by: The University of Chicago Press Stable URL: http://www.jstor.org/stable/30143442 Accessed: 25/06/2010 18:26 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=ucpress. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. The University of Chicago Press is collaborating with JSTOR to digitize, preserve and extend access to Infection Control and Hospital Epidemiology. -

Models, Techniques and Indicators of Quality Management Assessment in Manufacturing Industries Over the Past Decade
International Journal of Applied Research in Management and Economics ISSN 2538-8053 Models, Techniques and Indicators of Quality Management Assessment in Manufacturing Industries Over the Past Decade Atieh Tavakoli1, Amir Azizi2 1 Science and Research Branch, Islamic Azad university, Iran 2 Science and Research Branch, Islamic Azad university, Iran ([email protected]) ARTICLE INFO ABSTRACT Keywords: In this study 36 articles from reputable databases; "Scopus", Quality "TPBin", "google Scholor", and "Research Gate" have been Quality management considered. The focus of this paper was on manufacturing Quality Criteria industries key issues of quality management has been recited and Quality Assessment analyzed in this research ways. Therefore, on the basis of previous studies and evaluations, the involved components in the field of quality management have been investigated as the competitive advantages. During a period of 10 years since 2008 to 2018 as well as 1386 to 1396, various researches have been carried out in the field of manufacturing industries which indicate the importance of this managerial aspect and preference of top managers to improve production and optimization of the resources of their companies. Finally, models, common techniques and various measurement indicators of Quality Management along with important points and efficiency level of each of them have been identifies and presented. Introduction Service is a kind of product that has a significant share in the business and has enjoyed high qualitative and quantitative development in the third millennium. Services and quality have become a key tool in achieving competitive differentiation and promoting loyalty of customers. Today, the issue of products and services is a major problem that has affected all societies.