Infrared Spectral Markers for the Nephroprotective Effects of Ficus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determination of Mineral Content in the Ficus Deltoidea Leaves (Penentuan Kandungan Mineral Dalam Daun Ficus Deltoidea)
Jurnal Sains Kesihatan Malaysia 10 (2) 2012: 25-29 Kertas Asli/Original Article Determination of Mineral Content in The Ficus Deltoidea Leaves (Penentuan Kandungan Mineral dalam Daun Ficus Deltoidea) NIHAYAH MOHAMMAD, YONG KAR WEI & NUR FAIZAH ABU BAKAR ABSTRACT Ficus deltoidea leaves were widely used as a tea beverages in Malaysia with no information of its mineral content. Hence the mineral content of two species of Ficus deltoidea leaves were investigated. The dried leaves of F. deltoidea var. angustifolia and F. deltoidea var. deltoidea were acid digested and mineral elements of Na, Mg, K, Ca, Mn, Cr, Fe and Zn were determined using ICP–MS. Magnesium, potassium, sodium, manganese, iron and zinc were found to be present in the leaves of F. deltoidea var. angustifolia and F. deltoidea var. deltoidea. Concentration of magnesium (1934 mg/L), manganese (58.37 mg/L), iron (6.89 mg/L) and zinc (1.77 mg/L) in F. deltoidea var. deltoidea species were significantly (P < 0.05) higher than in F. deltoidea var. angustifolia species with concentration of 317 mg/L, 29.62 mg/L, 4.55 mg/L and 1.26 mg/L for magnesium, manganese, iron and zinc respectively. Meanwhile, concentration of sodium in F. deltoidea var. deltoidea species (3.13 mg/L) was found to be significantly (P < 0.05) lower than the concentration in F. deltoidea var. angustifolia species (9.11 mg/L). The finding showed that the leaves of F. deltoidea var. deltoidea has higher nutritional value than the leaves of F. deltoidea var. angustifolia. Leaves of Ficus deltoidea especially the F. -

Ficus Plants for Hawai'i Landscapes
Ornamentals and Flowers May 2007 OF-34 Ficus Plants for Hawai‘i Landscapes Melvin Wong Department of Tropical Plant and Soil Sciences icus, the fig genus, is part of the family Moraceae. Many ornamental Ficus species exist, and probably FJackfruit, breadfruit, cecropia, and mulberry also the most colorful one is Ficus elastica ‘Schrijveriana’ belong to this family. The objective of this publication (Fig. 8). Other Ficus elastica cultivars are ‘Abidjan’ (Fig. is to list the common fig plants used in landscaping and 9), ‘Decora’ (Fig. 10), ‘Asahi’ (Fig. 11), and ‘Gold’ (Fig. identify some of the species found in botanical gardens 12). Other banyan trees are Ficus lacor (pakur tree), in Hawai‘i. which can be seen at Foster Garden, O‘ahu, Ficus When we think of ficus (banyan) trees, we often think benjamina ‘Comosa’ (comosa benjamina, Fig. 13), of large trees with aerial roots. This is certainly accurate which can be seen on the UH Mänoa campus, Ficus for Ficus benghalensis (Indian banyan), Ficus micro neriifolia ‘Nemoralis’ (Fig. 14), which can be seen at carpa (Chinese banyan), and many others. Ficus the UH Lyon Arboretum, and Ficus rubiginosa (rusty benghalensis (Indian banyan, Fig. 1) are the large ban fig, Fig. 15). yans located in the center of Thomas Square in Hono In tropical rain forests, many birds and other animals lulu; the species is also featured in Disneyland (although feed on the fruits of different Ficus species. In Hawaii the tree there is artificial). Ficus microcarpa (Chinese this can be a negative feature, because large numbers of banyan, Fig. -

Recommendation of Native Species for the Reforestation of Degraded Land Using Live Staking in Antioquia and Caldas’ Departments (Colombia)
UNIVERSITÀ DEGLI STUDI DI PADOVA Department of Land, Environment Agriculture and Forestry Second Cycle Degree (MSc) in Forest Science Recommendation of native species for the reforestation of degraded land using live staking in Antioquia and Caldas’ Departments (Colombia) Supervisor Prof. Lorenzo Marini Co-supervisor Prof. Jaime Polanía Vorenberg Submitted by Alicia Pardo Moy Student N. 1218558 2019/2020 Summary Although Colombia is one of the countries with the greatest biodiversity in the world, it has many degraded areas due to agricultural and mining practices that have been carried out in recent decades. The high Andean forests are especially vulnerable to this type of soil erosion. The corporate purpose of ‘Reforestadora El Guásimo S.A.S.’ is to use wood from its plantations, but it also follows the parameters of the Forest Stewardship Council (FSC). For this reason, it carries out reforestation activities and programs and, very particularly, it is interested in carrying out ecological restoration processes in some critical sites. The study area is located between 2000 and 2750 masl and is considered a low Andean humid forest (bmh-MB). The average annual precipitation rate is 2057 mm and the average temperature is around 11 ºC. The soil has a sandy loam texture with low pH, which limits the amount of nutrients it can absorb. FAO (2014) suggests that around 10 genera are enough for a proper restoration. After a bibliographic revision, the genera chosen were Alchornea, Billia, Ficus, Inga, Meriania, Miconia, Ocotea, Protium, Prunus, Psidium, Symplocos, Tibouchina, and Weinmannia. Two inventories from 2013 and 2019, helped to determine different biodiversity indexes to check the survival of different species and to suggest the adequate characteristics of the individuals for a successful vegetative stakes reforestation. -

Tropical Garden Summer 2016
SUMMER 2016 Summer’s bounty in the tropics published by fairchild tropical botanic garden The Shop AT FAIRCHILD GARDENING SUPPLIES | UNIQUE TROPICAL GIFTS | APPAREL HOME DÉCOR | BOOKS | ECO-FRIENDLY AND FAIR-TraDE PRODUCTS ACCESSORIES | TROPICAL GOURMET FOODS | ORCHIDS AND MUCH MORE @ShopatFairchild SHOP HOURS: 9:00 A.M. - 5:30 P.M. SHOP ONLINE AT STORE.FAIRCHILDONLINE.COM contents FEATURES THE WORK OF CONSERVATION 18 37 THE FIGS OF FAIRCHILD DEPARTMENTS 4 FROM THE DIRECTOR 5 FROM THE CHIEF OPERATING OFFICER 7 SCHEDULE OF EVENTS 9 GET IN ON THE CONSERVATION 11 EXPLAINING 14 VIS-A-VIS VOLUNTEERS 17 THE ART IN GARTEN 18 CONSERVING 21 what’s in a name 28 what’s blooming 30 EXPLORING 37 PLANT COLLECTIONS 41 what’s in store 43 PLANT SOCIETIES EXPLORING THE WINDSWEPT 49 EDIBLE GARDENING ISLAND OF GREAT INAGUA 30 50 SOUTH FLORIDA GARDENING 53 BUG BEAT 59 BOOK REVIEW 60 FROM THE ARCHIVES 63 VISTAS 64 GARDEN VIEWS SUMMER 2016 3 from the director ummer at Fairchild is a time when we think about the future, a time for setting plans into motion for the years ahead. It’s when we add new plants to our landscape, launch research projects and develop training programs for our new recruits in botany. Summertime is when our best ideas begin to take shape. SSummertime is also when we keep an extra-vigilant eye on the warm Atlantic tropical waters. During hurricane season, we are constantly aware that everything we do, all of our dreams and hard work, are at risk of being knocked out whenever a storm spins toward South Florida. -

Influence of Rhizopheric H2O2 on Growth, Mineral Absorption, Root
agronomy Article Influence of Rhizopheric H2O2 on Growth, Mineral Absorption, Root Anatomy and Nematode Infection of Ficus deltoidea Nurul Hafiza Al Abadiyah Ralmi 1, Mohammad Moneruzzaman Khandaker 1,*, Khamsah Suryati Mohd 1, Ali Majrashi 2 , Ahmed M. Fallatah 3, Noor Afiza Badaluddin 1, Nornasuha Yusoff 1, Khairil Mahmud 4 , Mohamed Saifuddin 5, Normaniza Osman 6 and Zanariah Mohd Nor 1 1 School of Agriculture Science & Biotechnology, Faculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin, Besut Campus, Besut 22200, Malaysia; nurulhafi[email protected] (N.H.A.A.R.); [email protected] (K.S.M.); noorafi[email protected] (N.A.B.); [email protected] (N.Y.); [email protected] (Z.M.N.) 2 Department of Biology, Faculty of Science, Taif University, PO Box 11099, Taif 21944, Saudi Arabia; [email protected] 3 Department of Chemistry, College of Science, Taif University, PO Box 11099, Taif 21944, Saudi Arabia; [email protected] 4 Department of Crop Science, Faculty of Agriculture, Universiti Putra Malaysia, Seri Kembangan 43400, Malaysia; [email protected] 5 Computer and Communication Engineering (CCE), Faculty of Science, International Islamic University Chittagong, Kumira, Chittagong 4318, Bangladesh; [email protected] 6 Institute of Biological Sciences, Faculty of Science, Universiti Malaya, Kuala Lumpur 50603, Malaysia; Citation: Ralmi, N.H.A.A.; [email protected] Khandaker, M.M.; Mohd, K.S.; * Correspondence: [email protected]; Tel.: +609-6993450 Majrashi, A.; Fallatah, A.M.; Badaluddin, N.A.; Yusoff, N.; Abstract: Hydrogen peroxide (H2O2) is a broad-range chemical catalyst that is receiving rapidly Mahmud, K.; Saifuddin, M.; Osman, increasing attention recently due to its role as a signaling molecule in various plant physiological N.; et al. -

Leaf Morphological Variations and Heterophylly in Ficus Deltoidea Jack (Moraceae) (Variasi Morfologi Dan Heterofili Daun Dalamficus Deltoidea Jack (Moraceae))
Sains Malaysiana 41(5)(2012): 527-538 Leaf Morphological Variations and Heterophylly in Ficus deltoidea Jack (Moraceae) (Variasi Morfologi dan Heterofili Daun dalamFicus deltoidea Jack (Moraceae)) NASHRIYAH MAT*, NURRUL AKMAR ROSNI, NOR ZAIMAH AB RASHID, NORHASLINDA HARON, ZANARIAH MOHD NOR, NUR FATIHAH HASAN NUDIN, ABD GHANI YUNUS & ABDUL MANAF ALI ABSTRACT Six varieties of Ficus deltoidea Jack (Moraceae) showed leaf morphological variations through quantitative measurement on different plant parts. There were significant differences among six varieties studied by plant parts. The varieties studied include var. deltoidea Corner, var. angustifolia (Miq.) Corner, var. trengganuensis Corner, var. bilobata Corner, var. intermedia Corner, and var. kunstleri (King) Corner. The upper, middle and lower plant parts showed morphological variations in terms of leaf length, leaf width, leaf area and petiole length. Qualitative parameters also showed trends in morphological variations in terms of leaf shape, leaf base, leaf apex and leaf attachment. However, some qualitative parameters were not the recommended parameters to differentiate among varieties. On the other hand, leaf heterophylly has occurred in F. deltoidea because foliage of the young plant was different from the mature plant. Leaf heterophylly was observed in leaf shape and leaf apex parameters, whereby leaves from the lower plant parts were different from the upper and middle parts. The heterophylly in leaf shape was detected in varieties angustifolia, bilobata, intermedia and trengganuensis, whilst six varieties of F. deltoidea showed leaf apex heterophylly. Keywords: Ficus deltoidea Jack; leaf heterophylly; leaf morphological variations; plant parts; variety ABSTRAK Enam varieti Ficus deltoidea Jack (Moraceae) telah menunjukkan pelbagai variasi morfologi daun melalui ukuran kuantitatif pada bahagian pokok yang berlainan. -

Ficus Deltoidea Jack. ) Plant to Fertilization Treatments and Growth Activator 2
Scientific J. Flowers & Ornamental Plants www.ssfop.com/journal ISSN: 2356-7864 RESPONSE OF THE SLOW-GROWING MISTLETOE FIG (FICUS DELTOIDEA JACK. ) PLANT TO FERTILIZATION TREATMENTS AND GROWTH ACTIVATOR 2. HUMIC ACID LIQUID FERTILIZER TREATMENT Amal S. El-Fouly; Azza M. Abdel-Moneim and Hanan E. Ibrahim Ornamental Plants and Landscape Gardening Res. Dept., Hort. Res. Inst., ARC, Giza, Egypt. ABSTRACT: A series of pot experiments was conducted under plastic house at the nursery of Hort. Res. Inst., ARC, Giza, Egypt during 2012 and 2013 seasons to reveal the individual and combined effects of actosol (a humic acid NPK liquid fertilizer) when applied monthly as a foliar spray at the rates of 0.0, 2.5 and 5.0 ml/l and as a soil drench at the rates of 0.0, 10.0 and 15.0 ml/l on growth and chemical composition of mistletoe fig (Ficus deltoidea Jack.) transplants (6- months-old) grown in 20-cm-diameter plastic pots filled with about 2.5 kg of a mixture of sand, clay and peatmoss (1:1:1, v/v/v). The obtained results indicated that all vegetative and root growth parameters, the photosynthetic pigments (chlorophyll a, b and carotenoids) in the leaves, as well as N, P, K, Fe, Zn and Mn Scientific J. Flowers concentration in the leaves and roots were markedly improved in & Ornamental Plants, response to spraying or drenching with humic acid liquid fertilizer at 1(1):25-34 (2014). various levels, with the superiority of the combination between 5.0 ml/l Received: level as foliar spray and 10.0 ml/l level as soil drench, which gave, in 26/1/2014 general the highest records in the two seasons compared to control and all other treatments. -

Ficus Deltoidea Mistletoe Fig Moraceae
Ficus deltoidea Mistletoe fig Moraceae Forest Starr, Kim Starr, and Lloyd Loope United States Geological Survey--Biological Resources Division Haleakala Field Station, Maui, Hawai'i January, 2003 OVERVIEW F. deltoidea is one of many species of Ficus cultivated in various parts of the world as a houseplant or as an ornamental shrub. In Hawai'i, this species is not as commonly cultivated as other more popular figs, such as F. benjamina or F. elastica, and is known from just a few plantings. This species is not yet reproducing sexually because each Ficus species needs a specific pollinator wasp (Agaonidae) in order to reproduce and spread (Ramirez 1970) and the pollinator wasp for F. deltoidea has not yet been introduced to Hawai'i. The potential for Ficus species to spread in Hawai'i once their pollinator wasps are introduced has been demonstrated already and future naturalization of Ficus species could be prevented by preventing the introduction of pollinator wasps by adding them to the list of injurious animal species. This would prohibit the wasps from introduction and help to minimize the chance for this Ficus species to spread. In addition, F. deltoidea is not widely planted and removal of this species and adding it to the noxious weed list would also assist in prevention of future spread. TAXONOMY Family: Moraceae (Mulberry family) Latin name: Ficus deltoidea Jack (Bailey and Bailey 1976). Synonyms: Ficus diversifolia Blume (Bailey and Bailey 1976). Common names: Mistletoe fig, Mistletoe rubber plant (Bailey and Bailey 1976). Taxonomic notes: The genus Ficus is made up of about 1,000 species from pantropical and subtropical origins (Wagner et al. -

Ficus Plants for Hawai'i Landscapes
Ornamentals and Flowers May 2007 OF-34 Ficus Plants for Hawai‘i Landscapes Melvin Wong Department of Tropical Plant and Soil Sciences icus, the fig genus, is part of the family Moraceae. Many ornamental Ficus species exist, and probably FJackfruit, breadfruit, cecropia, and mulberry also the most colorful one is Ficus elastica ‘Schrijveriana’ belong to this family. The objective of this publication (Fig. 8). Other Ficus elastica cultivars are ‘Abidjan’ (Fig. is to list the common fig plants used in landscaping and 9), ‘Decora’ (Fig. 10), ‘Asahi’ (Fig. 11), and ‘Gold’ (Fig. identify some of the species found in botanical gardens 12). Other banyan trees are Ficus lacor (pakur tree), in Hawai‘i. which can be seen at Foster Garden, O‘ahu, Ficus When we think of ficus (banyan) trees, we often think benjamina ‘Comosa’ (comosa benjamina, Fig. 13), of large trees with aerial roots. This is certainly accurate which can be seen on the UH Mänoa campus, Ficus for Ficus benghalensis (Indian banyan), Ficus micro- neriifolia ‘Nemoralis’ (Fig. 14), which can be seen at carpa (Chinese banyan), and many others. Ficus the UH Lyon Arboretum, and Ficus rubiginosa (rusty benghalensis (Indian banyan, Fig. 1) are the large ban- fig, Fig. 15). yans located in the center of Thomas Square in Hono- In tropical rain forests, many birds and other animals lulu; the species is also featured in Disneyland (although feed on the fruits of different Ficus species. In Hawaii the tree there is artificial). Ficus microcarpa (Chinese this can be a negative feature, because large numbers of banyan, Fig. -
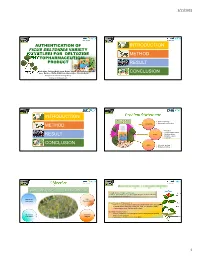
Authentication of Introduction Ficus Deltoidea Variety Kunstleri for Deltozide Method Phytopharmaceutical Product Result
1/11/2017 AUTHENTICATION OF INTRODUCTION FICUS DELTOIDEA VARIETY KUNSTLERI FOR DELTOZIDE METHOD PHYTOPHARMACEUTICAL PRODUCT RESULT Anee Suryani Sued, Zainah Adam, Fazliana Mohd Saaya, Bohari Yaacob, Hazlina Ahmad Hassali, Daryl Jesus Arapoc, Mohamed Zaffar Ali Mohamed Amiroudine, Rosniza Razali CONCLUSION Phytopharmaceutical Technology Group, Medical Technology Division INTRODUCTION Plant morphology Microscopic examination METHOD Authenticity Ash value Foreign organic matter Purity Moisture content RESULT Extractive values Crude fibre CONCLUSION Assay Chemical profiling Biological profiling unaided sense of sight, smell or taste with the aid of hand-lense (4-20X magnification) for visual identification use plant in whole / uncut form Macroscopy DNA observation fingerprinting Analysis based on attributes such as: Defined morphological and/or anatomical characteristics of the whole plant or individual plant parts (leaf, flower, fruit, seed, root & rhizome, bark) Characteristics colour, fracture, smell or taste Identification is achieved by: Positive comparison of morphological characteristics with authenticated Microscopy Chemical plant reference material examination analysis Authoritative technical reference description 1 1/11/2017 Use of higher magnification than provided by a hand lens Based on Literature Review Use of special light and staining techniques Identification based on microscopic observation of cell & tissue structure: Defined histological characteristics of plant parts (leaf, flower, fruit, seed, root & rhizome, bark) Defined staining or microscopic chemical reaction Identification is achieved by: kunstleri trengganuensis bilobata angustifolia deltoidea intermedia motleyana Use of a validated method Authoritative technical description of established microscopic characteristics Figure 1: The matured leaf shapes of Ficus deltoidea. Pictures showing the adaxial (upper) followed by abaxial (lower) surfaces. A - var. kunstleri, B- var. trengganuensis, C- var. bilobata, D- var. -

Ficus Deltoidea Leaves Methanol Extract Promote Wound Healing Activity in Mice
EurAsian Journal of BioSciences Eurasia J Biosci 14, 85-91 (2020) Ficus deltoidea leaves methanol extract promote wound healing activity in mice Retno Aryani 1*, Rudy Agung Nugroho 2, Hetty Manurung 3, Rani Mardayanti 2, Rudianto 2, Widha Prahastika 1, Auliana 3, Aulia Putri Bru Karo 2 1 Animal anatomy and Microtech, Department of Biology, Faculty of Mathematics and Natural Sciences, Mulawarman University, INDONESIA 2 Animal physiology, Development, and Molecular Laboratory, Department of Biology, Faculty of Mathematics and Natural Sciences, Mulawarman University, INDONESIA 3 Plant Physiology and Development Laboratory, Department of Biology, Faculty of Mathematics and Natural Sciences, Mulawarman University, INDONESIA *Corresponding author: [email protected] Abstract Wound healing is a normal process in skin tissue in response to injury. Ficus deltoidea leaves contain phytochemicals, which can play a role in wound healing. This study aimed to assess the wound healing activity of methanol extract of Ficus deltoidea leaves on artificial wounds in mice. In total 28 mice (2-3 months old, 20-30 g in weight) were randomly distributed into 7 treatment groups namely group I without treatment (negative control), group II were given povidone iodine 10% (positive control), group III was given a basic ointment and group IV-VII was treated with methanolic extract of Ficus deltoidea leaves with concentrations of 20, 40, 60 and 80% respectively. In all test animals, the wound was made with a length of 1 cm, and applied to the treatment according to the group, twice a day for 15 days. At the end of the treatment, wound healing activities were determined by measuring the percentage of wound contractions, Hydroxyproline estimates, and total new tissue DNA. -

Furnieles Nuñez Hector Javier.Pdf
ESTUDIO QUIMIOTAXONÓMICO Y EVALUACIÓN DE LA ACTIVIDAD ANTIOXIDANTE DE EXTRACTOS ETANÓLICOS FOLIARES DE CUATRO ESPECIES DEL GÉNERO Ficus L. (Moraceae), PLANETA RICA (CÓRDOBA- COLOMBIA). HÉCTOR JAVIER FURNIELES NÚÑEZ UNIVERSIDAD DE CÓRDOBA FACULTAD DE CIENCIAS BÁSICAS PROGRAMA DE BIOLOGÍA 2020 ESTUDIO QUIMIOTAXONÓMICO Y EVALUACIÓN DE LA ACTIVIDAD ANTIOXIDANTE DE EXTRACTOS ETANÓLICOS FOLIARES DE CUATRO ESPECIES DEL GÉNERO Ficus L. (Moraceae), PLANETA RICA (CÓRDOBA- COLOMBIA). HÉCTOR JAVIER FURNIELES NÚÑEZ Investigador Principal DIRECTORES JORGE ENRIQUE ARIAS, M.Sc. Docente programa de Biología Universidad de Córdoba MARY CECILIA MONTAÑO, PhD. Docente programa de Química Universidad de Córdoba UNIVERSIDAD DE CÓRDOBA FACULTAD DE CIENCIAS BÁSICAS PROGRAMA DE BIOLOGÍA 2020 AGRADECIMIENTOS Agradezco principalmente a Dios, por brindarme sabiduría, fuerza y constancia para lograr culminar con éxito este importante objetivo en mi vida. A mi familia por su apoyo y confianza, a la tía universal Nadys Núñez Ortega, gracias por tanto. A mis queridos padres Héctor Antonio Furnieles y Nerys Núñez Ortega, a mis hermanas Heidy Furnieles Núñez y Margarita Furnieles Núñez, por el apoyo incondicional y acompañamiento constante, aun en los momentos difíciles de esta hermosa travesía investigativa. A la Doctora Mary Montaño y al Magister Jorge Arias, por asumir la dirección de este proyecto, infinitamente agradecido por sus orientaciones, por compartir sus importantes y valiosos conocimientos científicos, que permitieron en mí el crecimiento profesional y científico con pensamiento crítico en las ciencias exactas. A los jurados, Doctora Rosalba Ruiz Vega y Magister Emmy Luz Sánchez, por sus valiosas recomendaciones. A mis compañeros de estudio, mil gracias por su amistad, hermandad y compañerismo. Gracias por animarme a seguir en las dificultades.