Silicones for Resin Modification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mechanical and Electrical Studies of Silicone Modified Polyurethane
Polymer Journal, Vol. 36, No. 10, pp. 848—855 (2004) Mechanical and Electrical Studies of Silicone Modified Polyurethane–Epoxy Intercrosslinked Networks y Arun ANAND PRABU and Muthukaruppan ALAGAR Department of Chemical Engineering, Anna University, Chennai-600 025, India (Received May 27, 2004; Accepted July 20, 2004; Published October 15, 2004) ABSTRACT: A series of intercrosslinked networks (ICNs) based on silicone modified polyurethane (PU)–epoxy resins were developed. In this study, epoxy resin (diglycidyl ether of bisphenol-A) was modified with PU prepolymer and hydroxyl-terminated polydimethylsiloxane (HTPDMS) using -aminopropyl triethoxysilane ( -APS) as silane cross linker and dibutyltindilaurate (DBTL) as catalyst to form ICNs. Aromatic polyamine adduct (A), diethylenetri- amine (B) and polyamidoamine (C) were used as epoxy curatives. The final products were obtained in the form of tough films. Changes in chemical structure during ICN formation, mechanical and electrical properties were investigated us- ing FT-IR spectra, tensile, impact and dielectric testing. The mechanical properties were enhanced with incorporation of PU (10 wt %) and silicone (10 wt %) due to the toughening of brittle epoxy matrices. Electrical properties showed a marginally decreasing trend with the incorporation of PU (0–20 wt %) influenced by the polar urethane linkages where- as silicone incorporation (10 wt %) showed an enhancement due to the presence of inorganic –Si–O–Si– linkage. Among the systems studied, the silicone (10 wt %) modified PU (10 wt %)–epoxy cured with ‘‘A’’ exhibited excellent mechanical and electrical characteristics and can be used as coatings and composites for industrial, electrical and ma- rine components. [DOI 10.1295/polymj.36.848] KEY WORDS Intercrosslinked Network / Epoxy / Polyurethane / Silicone / Coatings / Composites / Numerous polymeric resins based on epoxy, unsat- used in epoxy and polyurethane synthesis. -

United States Patent (19) 11 Patent Number: 5,077,324 Kistner Et Al
United States Patent (19) 11 Patent Number: 5,077,324 Kistner et al. 45 Date of Patent: Dec. 31, 1991 (54) REACTIVE SET AND MULTIPLE CHAMBER 3,915,297 0/1975 Rausch ................................ 206/29 CARTRIDGE AND PROCESS FOR 3,968,016 0/1976 Wisner ................................. 525/31 ADHESIVE ANCHORNG OF FASTENERS 4,105,114 0/1978 Knox et al. ......................... 405/261 4,343,921 0/1982 Piestert ................................ 52.5/531 INA BASE 4,58,283 0/1985 Gebauer et al. .................... 523/500 75 Inventors: Herbert Kistner, Freiburg; Christian 4,729,696 3/1988 Goto et al. ............................ 52.5/53 Weber, Emmendingen, both of Fed. Rep. of Germany OTHER PUBLICATIONS 73 Assignee: UPAT GmbH & Co., Emmendingen, Soviet "Inventions Illustrated', 1983, Week K11, Der Fed. Rep. of Germany went Publications, p. 36. Primary Examiner-Kriellion S. Morgan (21) Appl. No.: 315,892 Assistant Examiner-P. D. Niland 22 PCT Filed: Oct. 29, 1987 Attorney, Agent, or Firm-Baker & Daniels (86 PCT No.: PCT/DE87148700 57 ABSTRACT S371 Date: Feb. 14, 1989 A reactive composition for preparing synthetic resin bodies used in destructible multible-chamber cartridges S 102(e) Date: Feb. 14, 1989 and a process for anchoring a fastening element to the 87 PCT Pub. No.: WO88/03599 fastening base by introducing the destruction multiple chamber cartridges containing the components of a PCT Pub. Date: May 19, 1988 curable adhesive in a drilling in the fastening base. (30) Foreign Application Priority Data When the fastening element is driven into the hole, the cartridge is destroyed, the synthetic resin components Nov. 13, 1986 (DE) Fed. -
![Engineering Plastics[PDF:166KB]](https://docslib.b-cdn.net/cover/1063/engineering-plastics-pdf-166kb-711063.webp)
Engineering Plastics[PDF:166KB]
Daicel Group Businesses and Growth Strategies Engineering plastics TOPICS Contribution to Circular Economy with PET Bottle labels that Values Group Daicel for Framework Conceptual float on water While plastics are highly convenient and essential for modern higher specific gravity than water, do not float. Labels using society, they also become a cause of marine litter and TOPAS® COC (cyclic olefin copolymer), a material developed Supporting the sustainable global warming. To solve these issues, the easily recyclable by Polyplastics, fit this strategy because they do float in water, development of society by material for plastic beverage bottle labels was developed by enabling bottles and labels to be collected separately, simply Polyplastics. by placing bottle flakes in water and thereby accelerating offering plastics with special Since the raw materials used for plastic bottle bodies, the recycling process, while at the same time retaining the features such as mechanical caps, and labels differ, recycling plastic beverage bottles printability, shrinkability, and adhesiveness of conventional requires separating by resin type. In Europe, recycling labels. Moreover, although TOPAS® is different from strength and resistance to plants are equipped with a machine that removes labels polypropylene (PP) or polyethylene terephthalate (PET), labels Highlights Financial/Non-Financial heat and chemicals. from the bodies, and consumers must peel off the labels made of TOPAS® can be recycled as an olefin material. before disposal. Still, such approaches are rather ineffective As the demand for TOPAS® COC rapidly grows, it has in terms of both cost and results. As the search for a more become necessary to enhance our supply system for the efficient solution continues, a spotlight has been cast on a product. -

United States Patent Office Patented Dec
3,781,233 United States Patent Office Patented Dec. 25, 1973 1. 2 3,781,233 1 to 4 carbon atoms, hydroxyl or nitro groups or halo BLOWING AGENTS gen atoms and Erwin Muller, Leverkusen, Wolf-Dieter Wirth, Odenthal, X denotes a bond or a radical such as phenylene, naphthyl Johannes Blahak, Cologne, and Harry Rohr, Lever ene, diphenylene substituted with nitro or alkyl groups, kusen, Germany, assignors to Bayer Aktiengesellschaft, enediamine and N,N-dimethyl-N,N-dimethylene Leverkusen, Germany ylene, methylene dicyclohexylene, cyclohexylene, ethyl No Drawing. Filed May 25, 1972, Ser. No. 256,730 ene, dimethylene carbonate, N,N'-dimethylene-ethyl Claims priority, application Germany, May 26, 1971, enediamine and N,N'-dimethyl - N,N'-dimethylene P 21. 26 145.0 Int. C. C08 1/20 ethylenediamine or a radical obtained by removal of U.S. C. 260-2S R 13 Claims O two hydrogen atoms from a diphenylether or from ca as blowing agents for the production of cellular or porous ABSTRACT OF THE DISCLOSURE synthetic resin articles based on acrylonitrile, butadiene The invention relates to the use of bisbenzazimide com and styrene copolymers. pounds as blowing agents for the production of cellular The compounds according to the invention are suitable and porous articles from acrylonitrile, butadiene and for the production of foam plastics from thermoplastic styrene copolymers. The blowing agents do not liberate synthetic resins. The following are examples of com any corrosive, discoloring, malodorous or toxic decon pounds according to the invention: position products. 5,5'-dinitro-bisbenzazimide; 20 5,5'-dichloro-bisbenzazimide; This invention relates to the use of compounds of the 4,4'-dichloro-bisbenzazimide; bisbenzazimide series as blowing agents for the production 5,5'-dimethyl-bisbenzazimide; of cellular and porous articles based on acrylonitrile, 4,4'-dimethyl-bisbenzazimide; butadiene and styrene copolymers. -
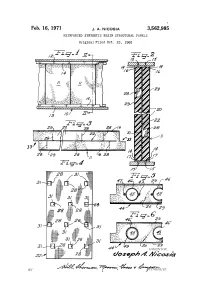
Oosea/31 Maasa by 1%-A- 7%Retc., 16-Ce Ya 47%Ily 3,562,985 United States Patent Office Patented Feb
Feb. 16, 1971 J. A. NICOSA 3,562,985 REINFORCED SYNTHETIC RESIN STRUCTURAL PANELS Original Filed Oct. 23, l965 RSSSSK2 S 12 INVENI ()R. oosea/31 Maasa BY 1%-a- 7%retc., 16-ce Ya 47%ily 3,562,985 United States Patent Office Patented Feb. 16, 1971 2 ing panels constructed in accordance with the present in 3,562,985 vention; REINFORCED SYNTHETIC RESEN FIG. 2 is an elevational side view taken along the lines STRUCTURAL PANELS Joseph A. Nicosia, 819 N. Thatcher Ave., II-II of FIG. 1; River Forest, III. 60305 FIG. 3 is a top plan view of the building board of Continuation of application Ser. No. 503,736, Oct. 23, FIGS. 2 and 3; 1965. This application Jan. 13, 1969, Ser. No. 793,225 FIG. 4 is a plan elevational view of part of the panel Int, C. E04c 2/46 of FIGS. 1-3; U.S. C. 52-24 4 Claims FIGS. 5 and 6 are top plan views similar to that of O FIG. 3, of two alternative constructions of the panels. As shown on the drawings, the structural boards and ABSTRACT OF THE DISCLOSURE panels of the present invention are made from any suit A building structure comprising a plurality of inter able foamed synthetic resin i.e. foamed polystyrene beads connecting barriers, said barriers comprising a plurality such as Pelaspan 8 and Pelaspan 18, polystyrene flakes, of interconnecting panels, said panels comprising a rigid epoxy resin, polyurethane of the polyester and polyure molded synthetic resin foam having embedded therein a thane of the polyether type. -

Manufacturing of Synthetic Resins with Formulation
Introduction Synthetic resins are materials with a property of interest that is similar to natural plant resins: they are viscous liquids that are capable of hardening permanently. Otherwise, chemically they are very different from the various resinous compounds secreted by plants. Synthetic resins comprise a large class of synthetic products that have some of the physical properties of natural resins but are different chemically. Synthetic resins are not clearly differentiated from plastics. www.entrepreneurindia.co In modern industry natural resins have been almost entirely replaced by synthetic resins, which are divided into two classes, thermoplastic resins, which remain plastic after heat treatment, and thermosetting resins, which become insoluble and infusible on heating. Thermoplastic resin softens repeatedly by heating. Thermosetting resin, on the other hand, hardens only once when heated. Thermoplastics produced by the local industry include Polystyrene (PS), Polyvinyl Chloride (PVC), Alkyds and Polyester Fiber, while those of thermosetting resins include Phthalic Anhydride, Aluminum Paste Resin, Adhesive Resin, Acrylic Resin Urea- and Phenol-Formaldehyde, and Colored Pellets. www.entrepreneurindia.co Thermosetting and thermoplastic resins respectively fall under two broad industrial categories. Thermosetting resins fall under the surface coating branch of the chemicals industry. Thermoplastic resins fall under plastic and plastic- based products. The surface coating chemicals branch includes the manufacture of paint, adhesives, printing ink, and specialty resins of the thermosetting type. Synthetic resins required pigments to be grinded, which provides excellent transparency and pigment wetting. The pigment concentrate must be let down with a synthetic resin that will provide the finished ink or coating attributes. www.entrepreneurindia.co These attributes may require a synthetic resin to have water resistance, alkali resistance and solvent resistance, as well as adhesion to the designated substrate. -

Innovative Silicone Solutions
Toronto Head Office, Research Lab and Plant Mississauga, Ontario, Canada Plant Siltech Corporation Siltech LLC (Personal Care Applications) 225 Wicksteed Avenue 1625 Lakes Parkway . Suite O Toronto . Ontario . Canada Lawrenceville . GA . USA M4H 1G5 30043 Telephone: 416.424.4567 Telephone: 678.442.0210 Facsimile: 416.424.3158 Facsimile: 678.442.9624 www.siltech.com ISO 9001:2008 Registered Aug/2016 INNOVATIVE SILICONE SOLUTIONS 14 Silicon : The 14th element in the periodic table; chemical symbol; Si, density = 2.33g/ml; molar mass = 28.09 g/mol; melting point = 1,420°C; boiling point = 3,265°C; electronic 2 2 Siltech is a privately owned business, managed and operated by the owners for more than 20 years. configuration [Ne]3s p ; metallic-looking; does not occur naturally in free form; in its combined We hope and believe that the pride we feel in this company channels its way through each of our form, accounts for 27.6% of the Earth’s crust; 2nd most abundant element on Earth after oxygen employees and to every customer. and one of the 10 most abundant elements in the solar system - 4.47 x 10 7 (rel. to [H] = 1 x 10 12). Siltech is a place where someone answers the phone when you call. It is a place where we feel passion about the quality of our products and realize that our livelihood depends on satisfying you, our customer. And it is a place where you can source products with confidence. Siltech develops and manufactures a full line of organo-functional silicone compounds and related specialties for a wide range of industrial and personal care applications, using our patented and otherwise proprietary technologies. -

Basic Silicone Chemistry – a Review
Editors Note: This edition of the Silicone Spectator is presenting a general article on Silicone Chemistry written in 1999. While the paper was written a long while ago the contents are still topical today. We hope you enjoy. Basic Silicone Chemistry – A Review Anthony J. O’Lenick, Jr. Siltech LLC Dacula, Ga. 30019 First Published: August 1999 This review has been written with the objective of supplying a working knowledge of the chemistry of silicone compounds to the practicing chemist. It has been divided into two parts, the first dealing with basic chemistry of silicones, and the second dealing with silicone based surfactants. Despite the fact that silicone compounds have been around for over fifty years, the chemistry of these materials remains elusive to the average formulating chemist. This is indeed unfortunate, since the chemistry of silicon atom and resulting silicone compounds is every bit as wide in scope and rich in content as the chemistry of the carbon atom and the resulting surfactant chemistry upon which it is based. Whilst the chemistry upon which surfactants are commonly based has been around for almost exactly the same period as that of the basic silicone chemistry, it is only in the past decade that the ability to use silicone as a hydrophobic material in the preparation of surfactants has been common. The recent trend to combine silicone, fatty and polyoxyalkylene moieties in the same molecule has resulted in a plethora of new compounds with new properties. Introduction Silicon is the 14th element in the periodic table. Although it does not occur naturally in free form, in its combined form it accounts for about 25% of the earth's crust. -

(Quartz) Silica, Sio2, Is a White Or Colorless Crystalline Compound
Silica (quartz) Silica, SiO2, is a white or colorless crystalline compound found mainly as quartz, sand, flint, and many other minerals. Silica is an important ingredient to manufacture a wide variety of materials. Quartz; Quartz is the most abundant silica mineral. Pure Quartz is colorless and transparent. It occurs in most igneous and practically all metamorphic and sedimentary rocks. It is used as a component of numerous industrial materials. Silicon (Si) has the atomic number 14 and is closely related to carbon. It is a relatively inert metalloid. Silicon is often used for microchips, glass, cement, and pottery. Silica is the most abundant mineral found in the crust of the earth. One of the most common uses of silica quarts is the manufacturer of glass. Silica is the fourteenth element on the periodic table. It can sometimes be found as the substance, quartz which is usually used in jewelry, test tubes, and when placed under pressure, generates an electrical charge. Quartz is the second most abundant mineral in the Earth's crust. It is a clear, glossy mineral with a hardness of 7 on the MOHS scale. Silica, Sa,is a component of glass and concrete. A form of Silica commonly known as quartz, Silica tetrahedra, is the second most common mineral in the earth's crust, it comes in many different forms. Silica is a compound of silicon and oxygen. Earth's outer crust contains 59% of this material. It has three major rock forms, which are quartz, tridymite, and cristobalite. Silica, commonly known in the form of quartz, is the dioxide form of silicon, SiO2. -

Silicone Rubber Science
Silicone Guide Power Chemical Corporation Limited Silicone Rubber Power Chemical Corporation Limited Silicone Rubber Quality & lower price is our commitment. Background Structure and Chemistry Silanes Siloxanes Classes of Silicone Rubbers Industrial Classifications Synthesis of Silicones Other Components in Silicones Curing Additives Fillers Other Additives Manufacture Liquid Silicone Rubbers Room Temperature Vulcanising (RTV) Rubbers Key Properties Advantages Disadvantages Thermal Stability Flexibility Resistance to Hydrocarbons, Oils and Solvents Gas Permeability Electrical Properties Applications Mechanical Engineering Electrical Engineering Medical - 2 - ©2011 Power Chemical Corporation Limited. SiSiB® is a registered trademark of PCC. www.PCC.asia www.SiSiB.com Power Chemical Fax: +86-25-8468-0091 Tel: +86-25-8468-0092 ISO9001 ISO14001 certificated June 2011 Silicone Rubber Quality & lower price is our commitment. Background Dumas predicted the existence of silicone rubbers ion 1840. However, it was not until 1872, that Ladenburg produced the first example, a very viscous oil, by reacting diethoxydiethysilane with water and trace amounts of acids. The first commercial grades were produced in 1943 by the Dow Chemical Company, with many other companies following shortly after. Back Structure and Chemistry Silanes Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the CH4 (methane) group, while that for silianes is SiH4 (silane) and the basic structure is SiH3(SiH2)nSiH3. These materials have the Si-Si-Si backbone and the resultant structures are named based on the number of silicon atoms in the chain i.e. silane, disilane, trisilane, tetrasilane etc etc. Furthermore, similar substitutions can take place, as is the case with hydrocarbons, e.g. -

Silicon Xanes Assessment of the Silicone Oil Content of Gems in Idps
Lunar and Planetary Science XLVIII (2017) 1059.pdf SILICON XANES ASSESSMENT OF THE SILICONE OIL CONTENT OF GEMS IN IDPS . G. J. Flynn 1, S. Wirick 2, A. L. Butterworth 3, Z. Gainsforth3, A. J. Westphal 3, B. Kaulich 4, T. Araki 4, M. Abyaneh 4, and T. Ty- liszczak 5. 1Dept. of Physics, SUNY-Plattsburgh, Plattsburgh, NY 12901 USA ( [email protected] ), 2Focused Beam Enterprises, Westhampton, NY 11977, 3Space Sciences Laboratory, University of California, Berke- ley, CA 94720, 4Diamond Light Source, Didcot, Oxfordshire OX11 0DE, UK, 5Advanced Light Source, 6 Cyclotron Rd., Berkeley, CA 94720 Introduction: Glass with Embedded Metal and copy, and Si x-ray absorption near-edge structure Sulfides (GEMS) is a major component in many chon- (XANES) spectroscopy provides a technique to assess dritic porous interplanetary dust particles (CP IDPs). the silicone oil content of GEMS. Barranco et al. [8] Based on the similarity of GEMS to the properties of performed Si-XANES on SiO xCyHz polymeric thin interstellar silicates, inferred from astronomical obser- films having compositions with different C/O atomic vations, Bradley [1] proposed that GEMS are surviv- ratios. They also measured Si-XANES spectra of Si- ing interstellar silicates. The GEMS in CP IDPs have carbide and SiO 2 and found that the energy of the onset been extensively studied over the past two decades. of the Si K absorption edge varied with the composi- Synchrotron-based infrared spectroscopy showed a tion from 1840 eV in SiC to 1844 eV in SiO 2. Because good match between the 10 micron Si-O absorption the Si-C and Si-O bonds absorb at significantly differ- feature of GEMS in CP IDPs and the silicates in two ent energies, Si-XANES is a particularly effective molecular clouds as well as in the regions surrounding technique to assess the ratio of Si-C to Si-O bonding at a T-Tauri star and a post-main sequence M star [2]. -

Expanded Plastics, Polyurethane, Polyamide and Polyester Fibres
Expanded Plastics, Polyurethane, Polyamide and Polyester Fibres (Polyepoxides and Epoxy Resins, Polyamides and Polyimides, Polyesters, Polyolefins, Polycondensation, Fiber Production, Prepolymer Production, Polyether Polyols with Epoxy Resins, Polyimides, Closed Cell Foamed Films and Sheets, Plastic Deformation, Closed Cell Polyimides) www.entrepreneurindia.co Introduction Expanded plastics are also known as foamed plastics or cellular plastics. Expanded plastics can be flexible, semi flexible, semi rigid or rigid. They can also be thermoplastic or thermosetting and can exist as open celled or closed celled materials. Expanded plastics may be prepared from most synthetic and many natural polymers. Most of the industrially important ones are made from polystyrene, polyvinyl chloride, polyurethanes and polyethylene, as well as from resins that derive from phenol, epoxy, etc. Polyurethane (PUR and PU) is polymer composed of a chain of organic units joined by carbamate (urethane) links. www.entrepreneurindia.co Polyurethane polymers are formed by combining two bi or higher functional monomers. One contains two or more isocyanate functional groups and the other contains two or more hydroxyl groups. More complicated monomers are also used. Polyurethane (PUR and PU) is a polymer composed of organic units joined by carbamate (urethane) links. While most polyurethanes are thermosetting polymers that do not melt when heated, thermoplastic polyurethanes are also available. www.entrepreneurindia.co Polyurethane polymers are traditionally and most commonly formed by reacting a di- or poly-isocyanate with a polyol. Both the isocyanates and polyols used to make polyurethanes contain, on average, two or more functional groups per molecule. A polyamide is a macromolecule with repeating units linked by amide bonds.