Alphabetical Listing of Drugs Acetazolamide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intraocular Pressure Rise After Phacoemulsification Prophylactic
British Journal of Ophthalmology 1995; 79: 809-813 809 Intraocular pressure rise after phacoemulsification Br J Ophthalmol: first published as 10.1136/bjo.79.9.809 on 1 September 1995. Downloaded from with posterior chamber lens implantation: effect of prophylactic medication, wound closure, and surgeon's experience Thomas G Bomer, Wolf-Dietrich A Lagreze, Jens Funk Abstract pressure rises usually occur between 6 and 8 Aims-A prospective clinical trial was hours after surgery.6 carried out to evaluate the effect of Various antiglaucomatous agents have been prophylactic medication, the technique of used to prevent the intraocular pressure rise wound closure, and the surgeon's experi- after cataract extraction. Oral acetazolamide15 16 ence on the intraocular pressure rise after and topical timolol'6-19 lowered the pressure cataract extraction. rise in the early period after intracapsular and Methods-In 100 eyes, the intraocular extracapsular cataract extraction. Levobunolol pressure was measured before as well as proved to be superior to timolol 4-7 hours 2-4, 5-7, and 22-24 hours after phaco- after extracapsular cataract extraction.20 Apra- emulsification and posterior chamber lens clonidine lowered the intraocular pressure rise implantation. Each of 25 patients received after uncomplicated phacoemulsification21 and either 1% topical apraclonidine, 0.5%/o extracapsular cataract extraction22 23 when topical levobunolol, 500 mg oral acetazo- given 30 minutes to 1 hour before surgery, lamide, or placebo. Forty four eyes were whereas immediate postoperative treatment operated with sclerocorneal sutureless with apraclonidine was ineffective.22 24 Miotics tunnel and 56 eyes with corneoscleral are frequently used to promote miosis and incision and suture. -

Brimonidine Tartrate; Brinzolamide
Contains Nonbinding Recommendations Draft Guidance on Brimonidine Tartrate ; Brinzolamide This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA, or the Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the Office of Generic Drugs. Active Ingredient: Brimonidine tartrate; Brinzolamide Dosage Form; Route: Suspension/drops; ophthalmic Strength: 0.2%; 1% Recommended Studies: One study Type of study: Bioequivalence (BE) study with clinical endpoint Design: Randomized (1:1), double-masked, parallel, two-arm, in vivo Strength: 0.2%; 1% Subjects: Males and females with chronic open angle glaucoma or ocular hypertension in both eyes. Additional comments: Specific recommendations are provided below. ______________________________________________________________________________ Analytes to measure (in appropriate biological fluid): Not applicable Bioequivalence based on (95% CI): Clinical endpoint Additional comments regarding the BE study with clinical endpoint: 1. The Office of Generic Drugs (OGD) recommends conducting a BE study with a clinical endpoint in the treatment of open angle glaucoma and ocular hypertension comparing the test product to the reference listed drug (RLD), each applied as one drop in both eyes three times daily at approximately 8:00 a.m., 4:00 p.m., and 10:00 p.m. for 42 days (6 weeks). 2. Inclusion criteria (the sponsor may add additional criteria): a. Male or nonpregnant females aged at least 18 years with chronic open angle glaucoma or ocular hypertension in both eyes b. -

Totalcare Medicaid 2017 Q2 GB
Preferred Drug List Molina Healthcare of New York, Inc. 2017 *Molina mandates the use of generic drugs, if available. Brand names listed are for reference only. THIS LIST IS SUBJECT TO CHANGE. You can get more information and updates to this document on our website at www.molinahealthcare.com MCD_CO_ESIPREFDRUGQ3_0817_08/27/2017 6025633NY0417 Table of Contents Anti - Infectives....................................................................................................................................................3 Antineoplastic / Immunosuppressant Drugs ......................................................................................................12 Autonomic / Cns Drugs, Neurology / Psych ......................................................................................................17 Cardiovascular, Hypertension / Lipids...............................................................................................................34 Dermatologicals/Topical Therapy......................................................................................................................41 Diagnostics / Miscellaneous Agents ..................................................................................................................56 Ear, Nose / Throat Medications..........................................................................................................................64 Endocrine/Diabetes ............................................................................................................................................66 -

Table 1. Glaucoma Medications: Mechanisms, Dosing and Precautions Brand Generic Mechanism of Action Dosage/Avg
OPTOMETRIC STUDY CENTER Table 1. Glaucoma Medications: Mechanisms, Dosing and Precautions Brand Generic Mechanism of Action Dosage/Avg. % Product Sizes Side Effects Warnings Reduction CHOLINERGIC AGENTS Direct Pilocarpine (generic) Pilocarpine 1%, 2%, 4% Increases trabecular outflow BID-QID/15-25% 15ml Headache, blurred vision, myopia, retinal detachment, bronchiole constriction, Angle closure, shortness of breath, retinal narrowing of angle detachment Indirect Phospholine Iodide (Pfizer) Echothiophate iodide 0.125% Increases trabecular outflow QD-BID/15-25% 5ml Same as above plus cataractogenic iris cysts in children, pupillary block, Same as above, plus avoid prior to any increased paralysis with succinylcholine general anesthetic procedure ALPHA-2 AGONISTS Alphagan P (Allergan) Brimonidine tartrate 0.1%, 0.15% with Purite Decreases aqueous production, increases BID-TID/up to 26% 5ml, 10ml, 15ml Dry mouth, hypotension, bradycardia, follicular conjunctivitis, ocular irritation, Monitor for shortness of breath, dizziness, preservative uveoscleral outflow pruritus, dermatitis, conjunctival blanching, eyelid retraction, mydriasis, drug ocular redness and itching, fatigue allergy Brimonidine tartrate Brimonidine tartrate 0.15%, 0.2% Same as above Same as above 5ml, 10ml Same as above Same as above (generic) Iopidine (Novartis) Apraclonidine 0.5% Decreases aqueous production BID-TID/up to 25% 5ml, 10ml Same as above but higher drug allergy (40%) Same as above BETA-BLOCKERS Non-selective Betagan (Allergan) Levobunolol 0.25%, 0.5% Decreases -

NEW ZEALAND DATA SHEET 1. PRODUCT NAME IOPIDINE® (Apraclonidine Hydrochloride) Eye Drops 0.5%
NEW ZEALAND DATA SHEET 1. PRODUCT NAME IOPIDINE® (apraclonidine hydrochloride) Eye Drops 0.5%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of Iopidine Eye Drops 0.5% contains apraclonidine hydrochloride 5.75 mg, equivalent to apraclonidine base 5 mg. Excipient with known effect Benzalkonium chloride 0.1 mg per 1 mL as a preservative. For the full list of excipients, see section 6.1. 2. PHARMACEUTICAL FORM Eye drops, solution, sterile, isotonic. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications Iopidine Eye Drops 0.5% are indicated for short-term adjunctive therapy in patients on maximally tolerated medical therapy who require additional IOP reduction. Patients on maximally tolerated medical therapy who are treated with Iopidine Eye Drops 0.5% to delay surgery should have frequent follow up examinations and treatment should be discontinued if the intraocular pressure rises significantly. The addition of Iopidine Eye Drops 0.5% to patients already using two aqueous suppressing drugs (i.e. beta-blocker plus carbonic anhydrase inhibitor) as part of their maximally tolerated medical therapy may not provide additional benefit. This is because apraclonidine is an aqueous-suppressing drug and the addition of a third aqueous suppressant may not significantly reduce IOP. The IOP-lowering efficacy of Iopidine Eye Drops 0.5% diminishes over time in some patients. This loss of effect, or tachyphylaxis, appears to be an individual occurrence with a variable time of onset and should be closely monitored. The benefit for most patients is less than one month. 4.2. Dose and method of administration Dose One drop of Iopidine Eye Drops 0.5% should be instilled into the affected eye(s) three times per day. -

Pharmacology of Ophthalmic Agents
Ophthalmic Pharmacology Richard Alan Lewis M.D., M.S., PHARMACOLOGY FOPS PHARMACOKINETICS OF Professor, Departments of Ophthalmology, • The study of the absorption, OPHTHALMIC Medicine, Pediatrics, and Molecular distribution, metabolism, AGENTS and Human Genetics and excretion of a drug or and the National School of Tropical agent Introduction and Review Medicine Houston, Texas PHARMACOKINETICS Factors Affecting Drug Penetration Factors Affecting Drug Penetration into Ocular Tissues • A drug can be delivered to ocular tissue: into Ocular Tissues – Locally: • Drug concentration and solubility: The higher the concentration the better the penetration, • Surfactants: The preservatives in ocular • Eye drop but limited by reflex tearing. preparations alter cell membrane in the cornea • Ointment and increase drug permeability, e.g., • Viscosity: Addition of methylcellulose and benzalkonium and thiomersal • Periocular injection polyvinyl alcohol increases drug penetration by • pH: The normal tear pH is 7.4; if the drug pH is • Intraocular injection increasing the contact time with the cornea and altering corneal epithelium. much different, it will cause reflex tearing. – Systemically: • Lipid solubility: Because of the lipid rich • Drug tonicity: When an alkaloid drug is put in • Orally environment of the epithelial cell membranes, relatively alkaloid medium, the proportion of the uncharged form will increase, thus more • IM the higher lipid solubility, the more the penetration. • IV penetration. FLUORESCEIN FLUORESCEIN Chemistry Dosage ● C20H1205, brown crystal ● Adults: 500-750 mg IV ● M.W. 322.3 e.g., 3 cc 25% solution ● Peak absorption 485-500 nm. 5 cc 10% solution ● Peak emission 520-530 nm. ● Children: 1.5-2.5 mg/kg IV Richard Alan Lewis, M.D., M.S. -

Hyperlinked Region IX SOP for MWLCEMS
2 Region IX 2 0 0 STANDARD OPERATING PROCEDURES/ 1 1 STANDING MEDICAL ORDERS 9 9 McHenry Western Lake County EMS System Healthcare delivery requires structure (people, equipment, education) and process (policies, protocols, procedures) that, when integrated, produce a system (programs, organizations, cultures) that leads to optimal outcomes (patient survival and safety, quality, satisfaction). An effective system of care comprises all of these elements—structure, process, system, and patient outcomes—in a framework of continuous quality improvement (AHA, 2015). These protocols have been developed and approved through a collaborative process involving the Advocate Lutheran General; Greater Elgin Area, McHenry Western Lake County, Northwest Community, Amita Saint Joseph Hospital, and Southern Fox Valley EMS Systems to reduce variation in practice and establish a Region-wide System of care. They shall be used: as the written practice guidelines/pathways of care approved by the EMS Medical Directors (EMS MDs) to be initiated by System EMS personnel for off-line medical control. as the standing medical orders to be used by Emergency Communications Registered Nurses (ECRNs) when providing on-line medical control (OLMC). in medium to large scale multiple patient incidents, given that the usual and customary forms of communication are contraindicated as specified in the Region IX disaster plan. System members are authorized to implement these orders to their scope of practice. OLMC communication shall be established without endangering the patient. Under no circumstances shall emergency prehospital care be delayed while attempting to establish contact with a hospital. In the event that communications cannot be established, EMS personnel shall continue to provide care to the degree authorized by their license, these protocols, drugs/equipment available, and their scope of practice granted by the EMS MD in that System. -

0Bcore Safety Profile
Core Safety Profile Active substance: Levobunolol Pharmaceutical form(s)/strength: Eye drops solution/ 0,1%; 0,25%; 0,5%; 0,5% UD P-RMS: CZ/H/PSUR/0006/001 Date of FAR: 26.05.2009 4.2 Posology and method of administration Adults (including the elderly) Country specific posology and method of administration to be included. Children /.../ is not recommended for use in children due to lack of safety and efficacy data. If required, /.../ may be used with other agents to lower intra-ocular pressure. The use of two topical beta-adrenergic blocking agents is not recommended (see section 4.4). Intraocular pressure should be measured approximately four weeks after starting treatment with /.../ as a return to normal ocular pressure can take a few weeks. As with any eye drops, to reduce possible systemic absorption, it is recommended that the lachrymal sac is compressed at the medial canthus (punctual occlusion) for one minute. This should be performed immediately following the instillation of each drop. Transfer from other beta-blocking treatment When another beta blocking agent is being used treatment must be discontinued after a full day of therapy. Start treatment with /.../ the next day with X drop of /.../ topically applied into the conjunctival sac in the affected eye(s). If /.../ is to replace a combination of anti-glaucoma products, only a single product should be removed at a time. Use in renal and hepatic impairment Levobunolol hydrochloride has not been studied in patients with hepatic or renal impairment. Therefore, caution should be used in treating such patients (see section 4.4). -

Pharmaceuticals Appendix
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ADAPALENE 106685-40-9 ABANOQUIL 90402-40-7 ADAPROLOL 101479-70-3 ABECARNIL 111841-85-1 ADEMETIONINE 17176-17-9 ABLUKAST 96566-25-5 ADENOSINE PHOSPHATE 61-19-8 ABUNIDAZOLE 91017-58-2 ADIBENDAN 100510-33-6 ACADESINE 2627-69-2 ADICILLIN 525-94-0 ACAMPROSATE 77337-76-9 ADIMOLOL 78459-19-5 ACAPRAZINE 55485-20-6 ADINAZOLAM 37115-32-5 ACARBOSE 56180-94-0 ADIPHENINE 64-95-9 ACEBROCHOL 514-50-1 ADIPIODONE 606-17-7 ACEBURIC ACID 26976-72-7 ADITEREN 56066-19-4 ACEBUTOLOL 37517-30-9 ADITOPRIME 56066-63-8 ACECAINIDE 32795-44-1 ADOSOPINE 88124-26-9 ACECARBROMAL 77-66-7 ADOZELESIN 110314-48-2 ACECLIDINE 827-61-2 ADRAFINIL 63547-13-7 ACECLOFENAC 89796-99-6 ADRENALONE 99-45-6 ACEDAPSONE 77-46-3 AFALANINE 2901-75-9 ACEDIASULFONE SODIUM 127-60-6 AFLOQUALONE 56287-74-2 ACEDOBEN 556-08-1 AFUROLOL 65776-67-2 ACEFLURANOL 80595-73-9 AGANODINE 86696-87-9 ACEFURTIAMINE 10072-48-7 AKLOMIDE 3011-89-0 ACEFYLLINE CLOFIBROL 70788-27-1 -

(12) Patent Application Publication (10) Pub. No.: US 2002/0187986 A1 Horn (43) Pub
US 2002O187986A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0187986 A1 Horn (43) Pub. Date: Dec. 12, 2002 (54) OPHTHALMIC FORMULATION WHICH Related U.S. Application Data MODULATES DILATION (62) Division of application No. 09/710,758, filed on Nov. (76) Inventor: Gerald Horn, Deerfield, IL (US) 8, 2000, now Pat. No. 6,420,407. Publication Classification Correspondence Address: Bell, Boyd & Lloyd LLC (51) Int. Cl." .................. A61K 31/517; A61K 31/4745; P.O. BOX 1135 A61K 31/4168; A61K 31/4164 Chicago, IL 60690 (US) (52) U.S. Cl. ..................... 514/252.17; 514/283; 514/401 (57) ABSTRACT (21) Appl. No.: 10/165,459 An ophthalmic formulation is disclosed which reduces dila (22) Filed: Jun. 7, 2002 tion in dim light and reduces redness. US 2002/0187986 A1 Dec. 12, 2002 OPHTHALMC FORMULATION WHICH 0007 A formulation for optimizing pupil size in extreme MODULATES DILATION lighting conditions is disclosed. The formulation is prefer ably a Solution of the type used in an artificial tear formu FIELD OF THE INVENTION lation having dissolved therein a therapeutically effective 0001. The present invention relates to a composition amount of a compound characterized by its ability to reduce formulated and administered to a human eye to reduce dilation of the eye, particularly in dim light. The compound dilation and redness. generally interferes with a natural biochemical reaction which results in the stimulation of the dilator muscles of the BACKGROUND OF THE INVENTION eye. The formulation is preferably further comprised of a compound which reduces eye redness, e.g. -

Sustained-Release Compositions Containing Cation Exchange Resins and Polycarboxylic Polymers
~" ' Nil II II II II Ml Ml INI MINI II J European Patent Office *»%r\ n » © Publication number: 0 429 732 B1 Office_„. europeen des brevets © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 16.03.94 © Int. CI.5: A61 K 9/06, A61 K 9/1 8, A61 K 47/32 © Application number: 89312590.6 @ Date of filing: 01.12.89 © Sustained-release compositions containing cation exchange resins and polycarboxylic polymers. @ Date of publication of application: (73) Proprietor: ALCON LABORATORIES, INC. 05.06.91 Bulletin 91/23 6201 South Freeway Fort Worth Texas 76107(US) © Publication of the grant of the patent: 16.03.94 Bulletin 94/11 @ Inventor: Janl, Rajni 4621 Briarhaven Road © Designated Contracting States: Fort Worth, Texas 76109(US) AT BE CH DE FR GB IT LI LU NL SE Inventor: Harris, Robert Gregg 3224 Westcliff Road W. © References cited: Fort Worth, Texas 76109(US) EP-A- 0 254 822 J.Pharm. SCI., vol. 60, no. 9, September 1971, © Representative: Jump, Timothy John Simon et pages 1343-1345 A. HEYD:"Polymer-drug in- al teraction: stability of aqueous gels contain- Venner Shipley & Co. ing neomycin sulfate" 20 Little Britain London EC1A 7DH (GB) 00 CM CO o> CM Note: Within nine months from the publication of the mention of the grant of the European patent, any person ® may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition CL shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee LU has been paid (Art. -
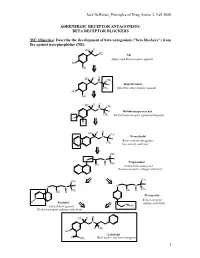
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted.