Corrosion of Rock Reinforcement in Underground Excavations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Northern Mining Eyes Mid-Term Gold Production
Research Note th 9 April 2015 Suite 5, Level 8, 99 York Street, Sydney NSW 2000 P: +61 2 9299 5001 I F: +61 2 9299 8001 [email protected] www.proactiveinvestors.com.au Northern Mining Limited Recommendation: Speculative Buy Northern Mining eyes mid-term gold production Sector: Materials o Northern Mining Ltd (ASX: NMI) is a Western Australian resource explorer that holds prime gold prospecting acreage located around Recommendation : Speculative Buy Kalgoorlie Boulder, on one of the world’s most productive gold belts. ASX Ticker Code: NMI o The acreage is known as the East Kalgoorlie Project and includes Share Price: $0.027 prospects at Blair North, Kanowna Lights and Snake Hill. 52 Week: - o Early stage exploration at Blair North has identified a structural High: $0.057 corridor that is 2 kilometres long that links the Company owned George’s Reward gold resource to an additional 1 kilometre of identified gold Low: $0.018 mineralisation at the northern end of the project area known as the Current Ordinary Northern Zone Deeps. Shares: 938.4M o Blair North forms the northern extension of the Cannon Gold Milestone shares (max): NIL Project mineralisation that is being jointly developed by Southern Gold (ASX:SAU) and Metals X (ASX:MLX), with Blair North constituting around Options: NIL 80% of the known gold strike line. Cash: $5.5M o The Cannon Gold Project currently includes shallow JORC gold Market Cap: $25.3M resources of 100,400 ounces that are contained within the southerly 20% Enterprise Value: $19.8M of the overall strike line, with the northern end of the Cannon open pit contiguous with gold resources owned by Northern Mining at the George’s Reward gold prospect. -
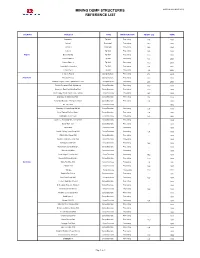
Mining Dump Structures Reference List
MINING DUMP STRUCTURES UPDATE OCTOBER 2012 REFERENCE LIST COUNTRY PROJECT TYPE MAIN FUNCTION HEIGHT [m] YEAR Luzamba Tip Wall Processing 11,4 1992 Catoca I Dump wall Processing 19,0 1995 Catoca II Dump wall Processing 16,0 1995 Catoca Tip Wall Processing 10,5 1996 Angola Escom Mining Tip Wall Processing 11,0 2002 Catoca Phase 1 Tip Wall Processing 13,6 2006 Catoca Phase 2 Tip Wall Processing 13,0 2006 Jopro 004 – Consulmet Tip Wall Processing 9,0 2007 Consulmet 2 Tip Wall Processing 9,0 2008 Veladero Project Dump structure Processing 27,8 2005 Argentina Pirquitas Project Dump structure Processing 18,0 2009 Veladero Project - Plant Expansion 85 KTPD Dump structure Processing 28,5 2009 Eastern Deepdale Pocket/Abutment Dump Structure Processing 16,0 1980 Saxonvale Raw Coal Handling Plant Dump Structure Processing 12,0 1981 Rom Hopper Walls Pacific Coal - Tarong Dump Structure Processing 19,5 1982 Boundary Hill Inpit Dump Wall - Dump Structure Processing 7,8 1982 Load Out Structure - Kangaroo Island Dump Structure Processing 6,0 1982 Mt. Tom Price Dump Structure - - 1982 Boundary Hill Inpit Dump Wall #2 Dump Structure Processing 8,0 1983 Kress Tipping Platform Stage I Dump Structure Processing 4,9 1984 Paddington Gold Project Dump Structure Processing 14,3 1984 Cork Tree Well Gold Mine Dump Wall Dump Structure Processing - 1985 Dump Wall - Cue Dump Structure Processing 8,3 1986 Telfer Mine Dump Structure Processing - 1986 Howick Colliery Temp Dump Wall Dump Structure Processing 8,4 1986 Wiluna Mine Dump Wall Dump Structure Processing - 1986 -

Codrus Minerals Limited (ASX:CDR, “Codrus”, Or the “Company”) Is Pleased to Report on Activities at Its Exploration Projects During the June 2021 Quarter
ASX ANNOUNCEMENT 22 July 2021 JUNE 2021 QUARTERLY REPORT Codrus lists on ASX after completing $8m IPO, commences maiden drilling program at Silver Swan South Highlights • Heavily oversubscribed $8m IPO at $0.20 completed on the 23rd June 2021. • 75m shares with $15m market cap and $7m enterprise value at listing. • Leveraged to exploration success with a high-quality project portfolio in Tier-1 locations. • Blackstone Minerals (ASX: BSX) remains cornerstone shareholder with 35m shares (46%). • Exploration programs planned to target projects in Australia and the USA. Codrus Minerals Limited (ASX:CDR, “Codrus”, or the “Company”) is pleased to report on activities at its exploration projects during the June 2021 Quarter. Corporate The official ASX listing of Codrus Minerals Limited occurred as planned on the 23rd of June 2021. A very well-supported Initial Public Offering was completed raising $8 million (before costs). The Company is now in a strong financial position to evaluate and explore its range of exciting projects in Tier-1 jurisdictions. Work Completed Since listing, the Company has focused its efforts on preparing for its maiden drilling program at the Silver Swan South Project (see below) and progressing planning for upcoming exploration across its high-quality project portfolio in the coming Quarter. Forward Plan During the September Quarter, exploration is planned to commence at all of the Company’s projects. WESTERN AUSTRALIAN PROJECTS The Company has three (3) projects in Western Australia. The Silver Swan South and Red Gate projects are located in the Kalgoorlie region and the Middle Creek Project is located near Nullagine in the Pilbara (Figure 1). -

Financial Report 30 June 2019
Codrus Minerals Limited (formerly known as Black Eagle (WA) Pty Ltd) ABN 17 600 818 157 Annual Report - 30 June 2019 Contents Corporate Directory 2 Directors’ Report 3 Auditor’s Independence Declaration 10 Financial Report 12 Directors’ Declaration 28 Independent Auditor’s Review Opinion 29 1 Corporate Directory Directors Auditors Andrew Radonjic Stantons International Shannan Bamforth Level 2 Jamie Byrde 1 Walker Avenue Stuart Owen WEST PERTH WA 6005 Company Secretary Jamie Byrde Bankers National Australia Bank Principal & Registered Office 50 St Georges Terrace Level 3, 24 Outram Street PERTH WA 6000 West Perth WA 6005 Telephone: (08) 9425 5217 Website Address Facsimile: (08) 6500 9982 www.codrusminerals.com.au Lawyers Steinepreis Paganin Lawyers & Consultants Level 4, 16 Milligan Street Perth WA 6000 Australia 2 Codrus Minerals Limited Directors' report 30 June 2019 The Directors present their report, together with the financial statements, on the consolidated entity (referred to hereafter as the 'Group') consisting of Codrus Minerals Limited (formerly known as Black Eagle (WA) Pty Ltd) (referred to hereafter as the 'Company' or 'Parent Entity') and the entities it controlled at the end of, or during, the year ended 30 June 2019. Directors The following persons were directors of Codrus Minerals Limited during the whole of the financial year and up to the date of this report, unless otherwise stated: Andrew Radonjic (Appointed on 1 August 2017) Shannan Bamforth (Appointed on 29 March 2021) Jamie Byrde (Appointed on 1 January 2021) Stuart Owen (Appointed on 1 January 2021; Resigned on 29 March 2021) Principal activities The principal activity of the Group during the year was mineral exploration. -

Role of the Safety Regulator
minesafeWESTERN AUSTRALIA Volume 19 no. 2 OCTOBER 2010 Role of the safety regulator ................................................. GETTING THE JOB DOne – SAFELY ................................................. ON THE ROAD WITH SAFE TOUGHNESS ................................................. SURFACE MINE EMERGENCY RESPONSE MineSafe vol. 19 no. 2 October 2010 COMPETITION 10 21 CONTENTS DEPARTMENTAL NEWS LEGAL NEWS INDUSTRY ACTIVITIES INDUSTRY PERFORMANCE 02 Safety reform progress report 13 What are you doing about 26 Miner’s Promise to leave a 04 What is the role of the mines your duty of care? lasting legacy 50 Mine helps Alex trial safety regulator? 28 Going underground in the city underground audits 51 Are you flashing? EXPLORATION SAFETY DIVISIONAL NEWS SURFACE MINE 14 Importance of exploration EMERGENCY RESPONSE CRUNCHING THE 08 Want to make a difference in notifications COMPETITION NUMBERS resources safety? 15 Raising dust at the Exploration 09 What you said about MineSafe Safety Roadshow 30 Mine site safety the real 52 Monthly mining workforce winner 10 Improving data handling with 53 Monthly exploration workforce 33 Realism the key to creating a the new SRS AXTAT+ 54 Distribution of safety and good scenario DANGEROUS GOODS health representatives as at SAFETY 36 Remote challenges for SIMON SAYS Newcrest Telfer 30 June 2010 16 Dealing with mineral 37 It’s tough being the new guys 38 No distance too far to hone 11 Bad news may get better concentrates classified as SIGNIFICANT INCIDENT results UN 3077 safety skills 18 Transport of -

RESERVES GROW 33% to 2MOZ at a COST of JUST A$50/OZ Resources up 350,000Oz to 9.25Moz with Four Recent Discoveries Yet to Be Included in the Estimate
RESERVES GROW 33% TO 2MOZ AT A COST OF JUST A$50/OZ Resources up 350,000oz to 9.25Moz with four recent discoveries yet to be included in the estimate KEY POINTS Northern Star’s highly successful exploration strategy has delivered a 33% increase (500,000oz) in Reserves to 2.0Moz after mining 611,000oz in FY16 Total Resources increased by 350,000oz to 9.25Moz, including an increase in Measured and Indicated Resources of 520,000oz to 4.9Moz Reserves added organically at a cost of A$50/oz – equal to ~10% of current market acquisition prices Five discoveries made in past year but only one included in latest Resource estimate due to 30 June cut-off date ASX ANNOUNCEMENT Significant Reserve increases at major mining centres: 28 July 2016 . Jundee up 21% to 720,000oz . Kanowna up 58% to 266,000oz Australian Securities . Millennium (100% Kundana) maiden Reserve of 205,000oz Exchange Code: NST . Hermes (Plutonic) maiden Reserve of 101,000oz Significant Resource estimates at a number of centres: Board of Directors . Kanowna up 20% to 1.4Moz Mr Chris Rowe Non-Executive Chairman . Raleigh (Kundana 50% JV) up 33% to 122,000oz at 42.2gpt . Hermes up 40% to 314,000oz (Plutonic) Mr Bill Beament Managing Director . Barkers (100% Kundana) Maiden Resource 137,000oz at 23gpt Mr Peter O’Connor FY17 exploration spend of A$62M to generate future mines, Non-Executive Director grow production and to follow up significant success of FY16 Mr John Fitzgerald Non-Executive Director Northern Star Resources Limited (ASX: NST) is pleased to advise that its strategy to grow production to 700,000 ounces a year in Ms Liza Carpene Company Secretary 2018 is well on track with a 33% increase in Reserves to two million ounces. -

2000 Annual Report
ABN 30 068 263 098 Heron Resources NL 21 Close Way, West Kalgoorlie PO Box 1280, Kalgoorlie, Western Australia 6430 Telephone: (08) 9091 9253 Facsimile: (08) 9091 9256 Email: [email protected] www.heronresources.com.au Heron, with the Rotary Club of Hannans and Rotary International supports the special education needs of East Kalgoorlie Primary School. One project involved minesite and bush excursions, with the children’s impressions captured in their art. Salena Abdullah’s painting was chosen to feature on the cover of this report. The title of Salena’s painting is “Binduli”. Reynolds Graphics, Kalgoorlie 11839 “My painting is of the manmade and natural environment. In the background is the revegetated land. In front is a road that leads to the mine. The colour represents the earth”. The accompanying photographic image of the Heron Goongarrie Project on the front cover can also be described by Salena’s words. Heron Resources NL Heron Resources NL Corporate Directory In the Community COMPANY SECRETARY The Artists: Ross James Arancini BBus, ACA - Company Secretary 110 Hensman Street, South Perth, Western Australia, 6151 Telephone: (08) 9367 1213 Facsimile: (08) 9367 7853 East Kalgoorlie Primary School Email: [email protected] Grades 3 to 7 REGISTERED AND PRINCIPAL OFFICE 21 Close Way, PO Box 1280, Kalgoorlie, Western Australia, 6430 Back Row, from left: Front Row, from left: Telephone: (08) 9091 9253 Facsimile: (08) 9091 9256 Derrick Sceghi, Kenneth Jackson, Natalia Tucker, Tony Champion, Email: [email protected] Wyvern Jackson, Kevin -

Groundwater Abstraction and Aquifer Response in the Roe Palaeodrainage (1990–2001)
Groundwater abstraction and aquifer response in the Roe Palaeodrainage (1990–2001) Department of Water Hydrogeological Record Series Report HG23 October 2007 Aquifer response in the Roe Palaeodrainage (1990-2001) Hydrogeological Record Series, HG23 Department of Water 168 St Georges Terrace Perth Western Australia 6000 www.water.wa.gov.au Telephone (08) 6364 7600 Facsimile (08) 8 6364 7601 For more information about this report, contact Seth Johnson Section Manager, Groundwater Investigations Department of Water PO Box K822 Perth WA 6842 Telephone (08) 6364 6897 Facsimile (08) 6364 6525 October 2007 ISSN 1329-542X ISSN 1834-9188 (pdf) ISBN 978-1-921094-72-9(Print) ISBN 978-1-921094-73-6 (PDF) A Cataloguing in Publication record for this report is available from the National Library of Australia Recommended reference The recommended reference for this publication is: Johnson, S.L., 2007, Groundwater abstraction and aquifer response in the Roe Palaeodrainage, Department of Water, Hydrogeological Record Series, HG 23. ii Department of Water Hydrogeological Record Series, HG23 Aquifer response in the Roe Palaeodrainage (1990-2001) Contents Summary.....................................................................................................................1 1 Introduction .............................................................................................................2 1.1 Overview ............................................................................................................................ 2 1.2 Purpose and -

(2000) Illustrating the Geometry of the Extensional Architecture of the System
The Lawlers Anticline and the Mt Margaret Anticline are located at the western and eastern margins of the Kalgoorlie and Kurnalpi Terranes respectively. Both folds are granite-cored and are associated with significant Late Basin preservation, and gold deposits. The initiation of the antiforms may have been during extension, with rising granite magma being emplaced into these incipient domes. The development of extensional shear zones and detachments within the granite and overlying greenstones tilted the country rocks so that when the Late Basins were deposited they ‘found’ a ‘D2’ pre-folded sequence. The intensity of this extensional event was sufficient to finally bring the lower-plate to the surface so that granitic detritus could be sourced and deposited both locally and afar. The presence of a layer parallel shear fabric along moderately dipping limbs of antiforms further support to the extension hypothesis since a steep axial-plane foliation which transects the layering would be expected in compression. Figure 61 shows that the partially radial pattern of extension away from granite dome cores during D3 observed at Lawlers is replicated on the regional scale. Figure 68: Schematic diagram from SRK (2000) illustrating the geometry of the extensional architecture of the system. The model has errors of fact such as assuming the Late Basins are the same age as the Kalgoorlie Sequence (Black Flags), but the overall picture is appealing. We have switched the view from on of ENE to one of north as we interpret that the fundamental polarity of extension to be E-W not NNW-SSE. Seismic reflection at Leonora and Pig Well – extensional formation of a Late Basin The project was fortunate to have access to publicly funded (GA-GSWA) seismic reflection data (01GASNY1) through the Leonora area (Fig. -

Downer in Western Australia
Downer in Western Australia Location 1 Albany 2 Argyle diamond mine 3 Barrow Island Legend 4 Brockman 2 and 4 iron ore mines 5 Bunbury 6 Burrup Peninsula Transport Rail Utilities Services Technology & Engineering, Mining 7 Canning Vale Services Communications Construction Services & Maintenance 8 Cannington 9 Cape Preston magnetite mine 10 Carosue Dam gold mine 11 Channar iron ore mine 12 Christmas Creek iron ore mine 13 Collie Argyle diamond mine 2 14 East Perth 15 Eastern Ridge iron ore mine Engineering Road Services Utilities Mining Rail 16 Granny Smith gold mine Construction & Maintenance 17 Gosnells Burrup Peninsula 6 18 Hope Downs iron ore mine Port Hedland 40 43 South Hedland Barrow Island 3 Karratha 25 19 Jimblebar iron ore mine Cape Preston magnetite mine 9 49 West Angelas Ore mine 20 Jundee gold mine Mesa A and J iron ore mines 37 Pannawonica iron ore mine 30 Telfer gold mine 47 21 Kalgoorlie Brockman 2 and 4 iron ore mines 4 Roy Hill iron ore mine 42 Yandi iron ore mines 50 22 Kambalda Christmas Creek iron ore mine 12 Marandoo iron ore mine 29 51 Yandicoogina iron ore mine 23 Kanowna Belle gold mine Tom Price iron ore mine 48 Newman 35 18 Hope Downs iron ore mine 24 Karara magnetite mine Paraburdoo iron ore mine 38 Channar iron ore mine 11 32 Mt Whaleback iron ore mine 25 Karratha WA Eastern Ridge Iron Ore mine 15 19 Jimblebar iron ore mine 26 KCGM gold mine 27 Kwinana Mt Keith nickel mine 31 28 Maddington 20 29 Marandoo iron ore mine Jundee gold mine WA 30 Mesa A and J iron ore mines 31 Mt -

For Personal Use Only Use Personal for of OTCQB: BLSTF; And
29 January 2021 Quarterly Report for the Period Ending 31 December 2020 Highlights • Blackstone Minerals (“Blackstone” or “the Company”) completed a Scoping Study for the development and restart of the Ta Khoa Nickel-Cu-PGE Project (“Ta Khoa Project”) in Vietnam. The Scoping Study is underpinned by the Ban Phuc Disseminated Sulfide (DSS) open pit mine, and integrates both upstream and downstream processing to produce a precursor product for Asia’s growing Lithium-ion battery industry (refer ASX announcement 14 October 2020); • The Company delivered some of the best intercepts to date, with successful infill drilling and extensional drilling results in the King Cobra Discovery Zone (KCZ) upgrading confidence in the Ban Phuc DSS deposit (refer ASX announcement 10 December 2020); • During the Dec’20 quarter Blackstone continued drilling focussed on delivering higher grade Massive Sulfide Vein (MSV) deposits to support ongoing studies and optimise cash flow; o The Company reported continued success at the Ban Chang prospect (refer ASX announcement 10 December 2020) o The Company’s in-house geophysics crew generated a new high priority target at the King Snake prospect, with drilling ongoing (refer ASX announcement 24 November 2020); o Blackstone continued drilling at Ta Cuong, using electromagnetic (EM) plates to test new MSV targets; • The Company strengthened its management team to drive development of Ta Khoa as a mine-to-market nickel business; • The Company announced the appointment of Mr Peter Plakidis as a Non-Executive Director and thanked Mr Steve Parsons, who resigned as Non-Executive Director, for his contributions; • Blackstone joined the US-based OTCQB Market to enhance visibility and accessibility to North American investors. -

1 Revision 1 1 Archaean Hydrothermal
1 revision 1 2 Archaean hydrothermal fluid modified zircons at Sunrise Dam and Kanowna Belle gold 3 deposits, Western Australia: Implications for post-magmatic fluid activity and ore 4 genesis 5 6 Rui Wanga*, Heejin Jeonb, Noreen J. Evansc 7 8 aState Key Laboratory of Geological Processes and Mineral Resources, and School of 9 Scientific Research, China University of Geosciences, Beijing 100083, China 10 bDepartment of Geosciences, Swedish Museum of Natural History, Box 50 007, SE-10405 11 Stockholm, Sweden 12 cSchool of Earth and Planetary Sciences, John de Laeter Centre, TIGeR, Curtin University, 13 Perth, WA 6102, Australia 14 15 16 17 18 *Corresponding author. 19 Email: [email protected] (Rui Wang) 20 Professor, School of Scientific Research, China University of Geosciences, Beijing 100083, 21 China 22 23 24 25 1 26 27 ABSTRACT 28 In order to further our knowledge of ore genesis in one of Australia’s preeminent ore 29 districts, we have completed a comprehensive geochemical study of ore-related porphyritic 30 intrusions from the Archaean Kanowna Belle and Sunrise Dam gold deposits (both > 10 31 Moz), Eastern Goldfields, Western Australia. Zircons (including samples from the newly 32 developed Velvet mine) with ages ranging from 2.8 to 2.2 Ga, were investigated for O-OH 33 isotopic signatures, trace element abundance, and U-Th-Pb compositions in order to elucidate 34 the nature of the magmatic source and ore-related fluid. These intrusions have similarly high 35 Sr/Y and La/Yb ratios to adakites from the Aleutian and Cook Islands, but lower Mg# values 36 and higher K2O contents, suggesting they were derived from partial melts in a thickened crust.