Harm Reduction Among Injecting Drug Users — Evidence of Effectiveness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bridging the Gap: a Practitioner's Guide to Harm Reduction in Drug
Bridging the Gap A Practitioner’s Guide to Harm Reduction in Drug Courts by Alejandra Garcia and Dave Lucas a Author Alejandra Garcia, MSW Center for Court Innovation Dave Lucas, MSW Center for Court Innovation Acknowledgements Bridging the Gap: A Practitioners Guide to Harm Reduction in Drug Courts represents an ambitious reimagining of drug court practices through a harm reduction lens. It was born of two intersecting health emergencies—COVID-19 and the overdose crisis—and a belief that this moment calls for challenging conversations and bold change. Against this backdrop, Bridging the Gap’s first aim is plain: to elevate the safety, dignity, and autonomy of current and future drug court participants. It is also an invitation to practitioners to revisit and reflect upon drug court principles from a new vantage point. There are some who see the core tenets of drug courts and harm reduction as antithetical. As such, disagreement is to be expected. Bridging the Gap aspires to be the beginning of an evolving discussion, not the final word. This publication would not have been possible without the support of Aaron Arnold, Annie Schachar, Karen Otis, Najah Magloire, Matt Watkins, and Julian Adler. We are deeply grateful for your thoughtful advice, careful edits, and encouraging words. A special thanks also to our designers, Samiha Amin Meah and Isaac Gertman. Bridging the Gap is dedicated to anyone working to make the world a safer place for people who use drugs. Thanks to all who approach this document with an open mind. For more information, email [email protected]. -

Harm Reduction Interventions in Substance Abuse Treatment
Prepared by: Karissa Hughes April 2018 Harm Reduction Interventions in Substance Abuse Treatment 1 Philosophy/Overview ● Harm reduction is a client-centered philosophy that engages clients in the process of behavior change even if they are not motivated to pursue abstinence from substance 2 use or refrain from engaging in other risk-taking behaviors as a treatment goal. ● Harm reduction challenges the traditional notion of abstinence as a universal treatment goal for problem substance use. It focuses on reducing the harm of drug use to the user and society (health, social, and economic consequences) for people unable or unwilling to stop using drugs rather than requiring abstinence as a condition of treatment. o Harm reduction strategies are community-based, user-driven, non-judgmental and address systems that isolate and marginalize individuals. o Harm reduction acknowledges that clients often seek out substance abuse treatment services to address risky injection practices and sexual risk-taking behavior, even when they are not interested in changing substance use patterns. ● Many people who use drugs prefer to use informal and non-clinical methods to reduce their drug consumption or reduce the risks associated with their drug use. Thus, harm reduction is a public health philosophy and service delivery model to reduce the risks of drug use while respecting the dignity and autonomy of individuals. o Harm reduction policies and practices emphasize the human right for the highest attainable standard for health of people who use drugs. o The approach is based on the belief that it is in both the user's and society's best interest to minimize the adverse consequences of drug use when the person is unable or unwilling to discontinue using. -

HIV and Substance Use October 2016
HIV and Substance Use October 2016 Fast Facts • Alcohol and other drugs can affect a person’s judgment and increase risk of getting or transmitting HIV. • In people living with HIV, substance use can worsen the overall consequences of HIV. • Social and structural factors make it difficult to prevent HIV among people who use substances. Substance use disorders, which are problematic patterns of using alcohol or another substance, such as crack cocaine, methamphetamine (“meth”), amyl nitrite (“poppers”), prescription opioids, and heroin, are closely associated with HIV and other sexually transmitted diseases. Injection drug use (IDU) can be a direct route of HIV transmission if people share needles, syringes, or other injection materials that are contaminated with HIV. However, drinking alcohol and ingesting, smoking, or inhaling drugs are also associated with increased risk for HIV. These substances alter judgment, which can lead to risky sexual behaviors (e.g., having sex without a condom, having multiple partners) that can make people more likely to get and transmit HIV. In people living with HIV, substance use can hasten disease progression, affect adherence to antiretroviral therapy (HIV medicine), and worsen the overall consequences of HIV. Commonly Used Substances and HIV Risk • Alcohol. Excessive alcohol consumption, notably binge drinking, can be an important risk factor for HIV because it is linked to risky sexual behaviors and, among people living with HIV, can hurt treatment outcomes. • Opioids. Opioids, a class of drugs that reduce pain, include both prescription drugs and heroin. They are associated with HIV risk behaviors such as needle sharing when injected and risky sex, and have been linked to a recent HIV outbreak. -
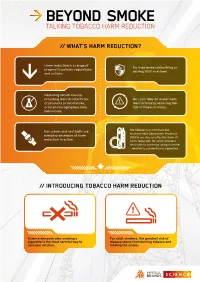
Talking Tobacco Harm Reduction
TALKING TOBACCO HARM REDUCTION // WHAT’S HARM REDUCTION? Harm reduction is a range of Do they make sunbathing or pragmatic policies, regulations driving 100% risk-free? and actions. Reducing health risks by providing less harmful forms No – but they do make them of products or substances, less harmful by reducing the or by encouraging less risky risk of illness or injury. behaviours. Sun cream and seat belts are We believe non-combustible nicotine Next Generation Products everyday examples of harm (NGPs) are also an effective form of reduction in action. harm reduction for adult smokers who wish to continue using nicotine – relative to conventional cigarettes. // INTRODUCING TOBACCO HARM REDUCTION Science demonstrates smoking a For adult smokers, the greatest risk of cigarette is the most harmful way to disease stems from burning tobacco and consume nicotine. inhaling the smoke. // WHAT IS THR? Tobacco smoke contains over 7000 The undisputed best action adult chemicals – nicotine is one of them. smokers can take to improve their Around 100 are classified by public health is to stop all tobacco and health experts as causes or potential nicotine use entirely, but many are not causes of smoking-related disease. interested or willing to take this step. Numerous public health bodies1 While the science suggests nicotine is believe transitioning to nicotine addictive and not risk-free, it’s neither products that are substantially less carcinogenic nor the primary cause of harmful than inhaled tobacco smoke smoking-related diseases. is their next best option – we agree. Contains 7000+ Contains chemicals, 100 significantly of them harmful fewer and lower or potentially levels of harmful harmful. -

Harm Reduction and Currently Illegal Drugs Implications for Nursing Policy, Practice, Education and Research
Harm Reduction and Currently Illegal Drugs Implications for Nursing Policy, Practice, Education and Research Discussion Paper This paper has been prepared by the Canadian Nurses Association (CNA) to stimulate dialogue on a particular topic or topics. The views and opinions expressed in this paper do not necessarily reflect the views of the CNA board of directors. CNA is the national professional voice of registered nurses in Canada. A federation of 11 provincial and territorial nursing associations and colleges representing 143,843 registered nurses, CNA advances the practice and profession of nursing to improve health outcomes and strengthen Canada’s publicly funded, not-for-profit health system. All rights reserved. No part of this document may be reproduced, stored in a retrieval system, or transcribed, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without written permission of the publisher. © Canadian Nurses Association 50 Driveway Ottawa, ON K2P 1E2 Tel.: 613-237-2133 or 1-800-361-8404 Fax: 613-237-3520 Website: www.cna-aiic.ca ISBN 978-1-55119-349-6 March 2011 TABLE OF CONTENTS Executive Summary ................................................................................................................. 3 Introduction ............................................................................................................................. 5 I. Illegal Drug Use in Canada .................................................................................................. 7 Health -

Advisory Council on the Misuse of Drugs
ACMD Advisory Council on the Misuse of Drugs Chair: Dr Owen Bowden-Jones Secretary: Zahi Sulaiman 1st Floor (NE), Peel Building 2 Marsham Street London SW1P 4DF Tel: 020 7035 1121 [email protected] Sarah Newton MP Minister for Vulnerability, Safeguarding and Countering Extremism Home Office 2 Marsham Street London SW1P 4DF 10 March 2017 Dear Minister, RE: Further advice on methylphenidate-related NPS In February 2016, my predecessor Professor Les Iversen wrote to the then minister for Preventing Abuse, Exploitation and Crime, requesting that the Temporary Class Drug Order (TCDO) on seven methylphenidate-related Novel Psychoactive Substances be re-laid for a further 12 months. This TCDO was re-laid until June 2017, to allow the Advisory Council on the Misuse of Drugs (ACMD) more time to collect the evidence required to provide further advice for full control under the Misuse of Drugs Act 1971. The ACMD believes that the TCDO has been effective in reducing the prevalence of these substances and that the TCDO level of control was proportionate in the interim. I am now pleased to present to you the ACMD’s further advice on this matter in the enclosed report. The ACMD’s recommendation for full control applies to the seven substances currently controlled under the TCDO and extends to an additional five closely-related substances. These five similar substances have subsequently appeared on markets following the TCDO and are included in this advice due to their potential for similar harms. Recommendation The ACMD recommends that the -

Needle Sharing Among Intravenous Drug Abusers: National and International Perspectives
Needle Sharing Among Intravenous Drug Abusers: National and International Perspectives U. S. DEPARTMENT OF HEALTH AND HUMAN SERVICES • Public Health Service • Alcohol, Drug Abuse, and Mental Health Administration Needle Sharing Among Intravenous Drug Abusers: National and International Perspectives Editors: Robert J. Battjes, D.S.W. Roy W. Pickens, Ph.D. Division of Clinical Research National Institute on Drug Abuse NIDA Research Monograph 80 1988 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, DC 20402 NIDA Research Monographs are prepared by the research divisions of the National Institute on Drug Abuse and published by its Office of Science. The primary objective of the series is to provide critical reviews of research problem areas and techniques, the content of state-of-the-art conferences, and integrative research reviews. Its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisors MARTIN W. ADLER, Ph.D. MARY L. JACOBSON Temple University School of Medrcrne National Federation of Parents for Philadelphia. Pennsylvania Drug-Free Youth Omaha, Nebraska SYDNEY ARCHER, Ph.D. Rensselaer Polytechnic Institute Troy, New York REESE T. JONES, M.D. Langley Porter Neuropsychiatric lnstitute RICHARD E. BELLEVILLE. Ph.D. San Francisco, California NB Associates, Health Sciences RockviIle, Maryland DENISE KANDEL, Ph.D. KARST J. BESTEMAN College of Physicians and Surgeons of Alcohol and Drug Problems Association Columbia University of North America New York, New York Washington, D. -

Treatment of Alzheimer's Disease and Blood–Brain Barrier Drug Delivery
pharmaceuticals Review Treatment of Alzheimer’s Disease and Blood–Brain Barrier Drug Delivery William M. Pardridge Department of Medicine, University of California, Los Angeles, CA 90024, USA; [email protected] Received: 24 October 2020; Accepted: 13 November 2020; Published: 16 November 2020 Abstract: Despite the enormity of the societal and health burdens caused by Alzheimer’s disease (AD), there have been no FDA approvals for new therapeutics for AD since 2003. This profound lack of progress in treatment of AD is due to dual problems, both related to the blood–brain barrier (BBB). First, 98% of small molecule drugs do not cross the BBB, and ~100% of biologic drugs do not cross the BBB, so BBB drug delivery technology is needed in AD drug development. Second, the pharmaceutical industry has not developed BBB drug delivery technology, which would enable industry to invent new therapeutics for AD that actually penetrate into brain parenchyma from blood. In 2020, less than 1% of all AD drug development projects use a BBB drug delivery technology. The pathogenesis of AD involves chronic neuro-inflammation, the progressive deposition of insoluble amyloid-beta or tau aggregates, and neural degeneration. New drugs that both attack these multiple sites in AD, and that have been coupled with BBB drug delivery technology, can lead to new and effective treatments of this serious disorder. Keywords: blood–brain barrier; brain drug delivery; drug targeting; endothelium; Alzheimer’s disease; therapeutic antibodies; neurotrophins; TNF inhibitors 1. Introduction Alzheimer’s Disease (AD) afflicts over 50 million people world-wide, and this health burden costs over 1% of global GDP [1]. -

Epidemics of HIV, HCV and Syphilis Infection Among Synthetic Drugs
www.nature.com/scientificreports OPEN Epidemics of HIV, HCV and syphilis infection among synthetic drugs only users, heroin-only users and Received: 7 November 2017 Accepted: 28 March 2018 poly-drug users in Southwest China Published: xx xx xxxx Shu Su1, Limin Mao 2, Jinxian Zhao3, Liang Chen3, Jun Jing4, Feng Cheng4 & Lei Zhang 1,4,5 The number of poly-drug users who mix use heroin and synthetic drugs (SD) is increasing worldwide. The objective of this study is to measure the risk factors for being infected with hepatitis C (HCV), human immunodefciency virus (HIV) and syphilis among SD-only users, heroin-only users and poly- drug users. A cross-sectional study was conducted in 2015 from a national HIV surveillance site in Southwest China, 447 poly-drug, 526 SD-only and 318 heroin-only users were recruited. Poly-drug users have higher drug-use frequency, higher rates of drug-sharing and unsafe sexual acts than other users (p < 0.05). About a third (36.7%) of poly-drug users experienced sexual arousal due to drug efects, which is higher than the rate among other drug users. Poly-drug users had the highest prevalence of HIV (10.5%) and syphilis (3.6%), but heroin-only users had the highest prevalence of HCV (66.0%) (all p < 0.05) among three groups. Logistic regression shows among poly-drug users, having sex following drug consumption and using drugs ≥1/day were the major risk factors for both HIV (Adjusted odds ratio (AOR) = 2.4, 95% CI [1.8–3.4]; 2.3, [1.6–3.1]) and syphilis infection (AOR = 4.1, [2.1–6.9]; 3.9, [1.8–5.4]). -

2.3 Harm Reduction – What Is It and Why Is It Useful?
SENSIBLE CANNABIS EDUCATION A Toolkit for Educating Youth 2.3 HARM REDUCTION – WHAT IS IT AND WHY IS IT USEFUL? By the end of this section, you will: 1. Understand what harm reduction is 2. Understand practical ways to reduce the harms associated with cannabis use, through both abstinence and the reduction of risky behaviours for youth who are already using cannabis WHAT IS HARM REDUCTION? “Taking a pragmatic approach to this generally understood phenomenon, harm reduction avoids taking a uniform stance that substance use is bad, but instead focuses on getting accurate and unbiased information on the harm of use to potential users, in order to help them make informed decisions about whether to use, and if they choose to use, what precautions to take to minimize their risk.”277 Harm reduction is a philosophy that underpins public health approaches to drugs and drug use, and attempts to reduce the harms of drug use without necessarily reducing drug use itself. Harm reduction acknowledges that there are inherent risks involved with a range of behaviours and that there are ways to reduce those risks. Harm reduction can also be understood in the context of a range of activities other than drug use, as simple as wearing sunscreen or wearing a helmet. REDUCING CANNABIS-RELATED HARMS In order to ensure cannabis education is suitable for all young people, discussing strategies to reduce the harms of cannabis use is of critical importance to supporting responsible and safe use among those youth who may choose to use cannabis. In 2017, the Canadian Research Initiative in Substance Misuse (CRISM) released an evidence- based guide on how to improve health and minimize risk for Canadians who use cannabis.278 The following discussion relies on CRISM’s “Lower-Risk Cannabis Use Guidelines” (LRCUG), however, it is tailored to youth based on feedback from our content committee and contributors. -

Methylphenidate-Based
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Secretary: Zahi Sulaiman 1st Floor (NE), Peel Building 2 Marsham Street London SW1P 4DF Tel: 020 7035 1121 [email protected] Minister of State for Crime Prevention Home Office 2 Marsham Street London SW1P 4DF 31 March 2015 Dear Minister, I am writing to recommend that you lay a temporary class drug order (TCDO) pursuant to section 2A of the Misuse of Drugs Act 1971 on a number of methylphenidate-based NPS: ethylphenidate, 3,4-dichloromethylphenidate (‘3,4- DCMP’), methylnaphthidate (‘HDMP-28’), isopropylphenidate (‘IPP’ or ‘IPPD’) and propylphenidate. Methylphenidate-based NPS Methylphenidate is a licensed stimulant pharmaceutical and is controlled in the UK as a Class B controlled drug. The methylphenidate-related materials being marketed as NPS have psychoactive effects so similar to the parent compound that they can be expected to present similar risks to users. Although ethylphenidate is by far the most widely available of this group, other variants are already in the market place. In the short term, to address the widespread availability of methylphenidate-based NPS and the associated problems which are being reported, the ACMD has considered the evidence on methylphenidate-based NPS and recommends control of these NPS by means of a TCDO. The attached report contains the ACMD’s consideration of the evidence concerning methylphenidate-based NPS. 1 In providing this advice, I would like to convey my thanks to Police Scotland, the National Programme on -

Reducing Cannabis Harms: a Guide for Ontario Campuses
Reducing cannabis harms: A guide for Ontario campuses About This Guide This guide explores issues related to cannabis use and provides readers with an overview of health approaches that can reduce the harms and risks associated with it. Any campus professional — whether faculty, academic advisor, counsellor, or student services professional — working with students who use cannabis will be able to refer to the guide for information. The first section will give you a better understanding of cannabis, the Ontario context, the substance use spectrum, as well as substance use disorders and problematic substance use. Section 2 looks at the reasons why students use or don’t use cannabis, the effects of cannabis use on the brain in adolescents and young adults, the link between cannabis use and mental health, the effects of language and stigma, and strategies that campus professionals can use to reduce harms when directly engaging with students. The final section provides concrete steps for developing a campus-wide cannabis-use framework to reduce harm. While the focus of this guide is on cannabis use by students, not campus staff or faculty, this is in no way meant to minimize the need to address the broader scope of mental health, substance use, and well- being on campuses, including among faculty and staff. Each post-secondary institution has unique strengths, circumstances, and needs. For this reason, while the broad areas addressed in this guide are relevant to all institutions, it is not meant to be prescriptive or to serve as legal advice pertaining to cannabis use legislation. As of its writing, the information in this guide is accurate and reflects current research and legislation.