NH COVID-19 Vaccination Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IHS Covid-19 Response 100 Day Review
INDIAN HEALTH SERVICE COVID-19 RESPONSE, 100 DAY REVIEW PLANNING SECTION Table of Contents Introduction ................................................................................................................................................. 2 Executive Summary .................................................................................................................................... 2 Summary of activities by aim ................................................................................................................. 3 Indian Health Service Response to COVID-19 ........................................................................................ 4 COVID-19 Funding ................................................................................................................................ 5 Aims and Strategic Objectives of the IHS Action Plan ....................................................................... 6 Aim 1: To Prevent the Spread of COVID-19 ....................................................................................... 7 Aim 2: To Detect Cases of COVID-19 ................................................................................................... 8 Aim 3: To Treat COVID-19 Cases and Sustain Regular Operations ............................................... 10 Aim 4: To Support the Indian Health System in the Recovery from COVID-19 ........................... 11 Aim 5: To Manage Resources ............................................................................................................. -

Number Games Full Book.Pdf
Should you read this book? If you think it is interesting that on September 11, 2001, Flight 77 reportedly hit the 77 foot tall Pentagon, in Washington D.C. on the 77th Meridian West, after taking off at 8:20 AM and crashing at 9:37 AM, 77 minutes later, then this book is for you. Furthermore, if you can comprehend that there is a code of numbers behind the letters of the English language, as simple as A, B, C is 1, 2, 3, and using this code reveals that phrases and names such as ‘September Eleventh’, ‘World Trade Center’ and ‘Order From Chaos’ equate to 77, this book is definitely for you. And please know, these are all facts, just the same as it is a fact that Pentagon construction began September 11, 1941, just prior to Pearl Harbor. Table of Contents 1 – Introduction to Gematria, the Language of The Cabal 2 – 1968, Year of the Coronavirus & 9/11 Master Plan 3 – 222 Months Later, From 9/11 to the Coronavirus Pandemic 4 – Event 201, The Jesuit Order, Anthony Fauci & Pope Francis 5 – Crimson Contagion Pandemic Exercise & New York Times 6 – Clade X Pandemic Exercise & the Pandemic 666 Days Later 7 – Operation Dark Winter & Mr. Bright’s “Darkest Winter” Warning 8 – Donald Trump’s Vaccine Plan, Operation Warp Speed 9 – H.R. 6666, Contact Tracing, ID2020 & the Big Tech Takeover 10 – Rockefeller’s 2010 Scenarios for the Future of Technology 11 – Bill Gates’ First Birthday on Jonas Salk’s 42nd... & Elvis 12 – Tom Hanks & the Use of Celebrity to Sell the Pandemic 13 – Nadia the Tiger, Tiger King & Year of the Tiger, 2022 14 – Coronavirus Predictive -

Flu Season: U.S
1 Written Testimony House Committee on Energy and Commerce, Subcommittee on Oversight and Investigations Flu Season: U.S. Public Health Preparedness and Response Statement of Robert Kadlec, MD, MTM&H, MS Assistant Secretary For Preparedness and Response For Release upon Delivery Expected at TBD December 4, 2019 2 Introduction Chairwoman DeGette, Ranking Member Guthrie, and distinguished Members of the Subcommittee, thank you for the opportunity to testify on our efforts to develop appropriate and effective medical countermeasures to mitigate a future pandemic influenza event. I am Dr. Bob Kadlec, the Assistant Secretary for Preparedness and Response (ASPR) at the Department of Health and Human Services (HHS). Today, I will provide background about how ASPR is partnering with the private sector to develop influenza vaccines, antivirals, and diagnostics to ensure we are as prepared for both seasonal as well as pandemic influenza. I will also provide an overview of the current challenges we face in preparedness due to dependence on active pharmaceutical ingredients (API) and other raw materials manufactured in other countries. Pandemic Influenza: a Costly National Security Threat Influenza has long posed a serious threat to human health. Seasonal influenza epidemics occur every year, leading to hundreds of thousands of hospitalizations and tens of thousands of deaths,1 and billions of dollars in economic loss. In the simplest of terms, the difference between seasonal influenza and a flu pandemic is that a pandemic occurs when a new flu virus emerges that humans have little or no immunity against, allowing the virus to spread easily from person to person worldwide. A pandemic influenza event could occur at any time, potentially claiming hundreds of thousands of lives. -

P2P Supply Chain Report
PRINCIPAL TO PRINCIPAL MAY 2021 PRINCIPAL TO PRINCIPAL GLOBAL SUPPLY CHAIN TASK FORCE REIMAGINING THE GLOBAL SUPPLY CHAIN POST COVID-19 PAGE 1 OF 32 PRINCIPAL TO PRINCIPAL TASK FORCE MEMBERS Co-Chairs: The Honorable Joe Crowley The Honorable Mike Rogers Members: Professor Ravi Anupindi, University of Michigan Professor Susan Helper, Case Western Reserve University 3M Tom Kelly, IDX Johnson & Johnson MITRE Corporation Siemens Government Technologies, Inc. Kristi Rogers, Principal to Principal Graduate Student Fellows (University of Michigan): David Horvath Avril Prakash Julie Sierks PAGE 2 OF 32 PRINCIPAL TO PRINCIPAL RECOMMENDATIONS SUMMARY The COVID-19 pandemic (“the pandemic”) is ushering in a new normal and we must grab this opportunity to evolve our national response because unfortunately, the next crisis is likely around the corner. Today we have an opportunity to put our American ingenuity and innovation to work to develop next-generation emergency response solutions, transform our supporting critical infrastructure, and strengthen our national and economic security. Our manufacturing and its supporting supply chains are not only core to our national response, but also our national and economic security. Given this, our national preparedness must receive the same attention and visibility as our national defense. During the pandemic, numerous agencies with overlapping and often duplicative authorities muddled the government’s response capability. It was evident that appropriate planning and coordination did not occur. Further, despite the existence of the Department of Homeland Security, it remains unclear which federal agency and/or department should have primary authority and accountability for national disaster preparedness and response. Clear lines of authority and communication are necessary to prepare, respond, galvanize the private sector, and create both a whole-of-government and a whole-of-nation approach. -

Using Federal Law to Measure Need, Stimulate Production
CHAPTER 23 • ASSURING ESSENTIAL MEDICAL SUPPLIES DURING A PANDEMIC: USING FEDERAL LAW TO MEASURE NEED, STIMULATE PRODUCTION, & COORDINATE DISTRIBUTION Assuring Essential Medical Supplies During a Pandemic: Using Federal Law to Measure Need, Stimulate Production, and Coordinate Distribution Evan Anderson, JD, PhD, University of Pennsylvania; Scott Burris, JD, Temple University Beasley School of Law SUMMARY. The global COVID-19 pandemic has temporarily increased demand for basic medical equipment and supplies, and disrupted global supply chains. Governments at all levels and the private sector have found themselves scrambling — and often competing — for the supplies they need. Federal law anticipates that emergencies can generate this kind of sudden demand for medical equipment. Federal agencies not only have ample legal authority to respond to shortages, but also the duty and the authority to prepare for emergencies by planning, supply-chain monitoring, investment and partnership with the private sector, and stockpiling. Perhaps the most important federal law for preventing and ameliorating shortages, and the primary focus of this Chapter, is the federal Defense Production Act (DPA). The DPA provides a menu of powers to stimulate production, strengthen supply chains, coordinate expertise, and resolve market failures. Although the shortfall in personal protective equipment and other basic medical equipment was anticipated by planners and demonstrated in simulation exercises, federal action to address the problem in the face of the pandemic -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

Political Polarization and the Dissemination of Misinformation: the United States Pandemic Response As a Cautionary Tale
The University of Maine DigitalCommons@UMaine Honors College Spring 5-2021 Political Polarization and the Dissemination of Misinformation: the United States Pandemic Response as a Cautionary Tale Mary Giglio Follow this and additional works at: https://digitalcommons.library.umaine.edu/honors Part of the American Politics Commons This Honors Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Honors College by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. POLITICAL POLARIZATION AND THE DISSEMINATION OF MISINFORMATION: THE UNITED STATES PANDEMIC RESPONSE AS A CAUTIONARY TALE by Mary K. Giglio A Thesis Submitted in Partial Fulfillment of the Requirements for a Dual-Degree with Honors (International Affairs and Political Science) The Honors College The University of Maine May 2021 Advisory Committee: Richard Powell, Professor of Political Science, Advisor Mark Brewer, Professor of Political Science, Honors College Lora Pitman, Adjunct Assistant Professor of Political Science Asif Nawaz, Assistant Professor of History and International Affairs Zachary Rockwell Ludington, Assistant Professor of Spanish i ABSTRACT This thesis discusses the failings of the United States response to the COVID-19 pandemic, and how it has been shaped by the nation’s intense political polarization and the widespread dissemination of misinformation. In this thesis, I critically examine the government’s initial response to the pandemic, including its lack of preparedness and the ineffectiveness of its eventual policies. I also attempt to explain the influence of political polarization on the states, resulting in congressional gridlock, as well as wildly varying policies regarding lockdowns and mask mandates. -
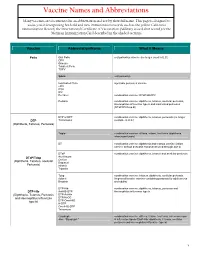
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

Adult Immunizations
Guidelines for Clinical Care Ambulatory Immunizations Guideline Team Adult Immunizations Team Leads Susan F Engert, MD, MPH Population: Adults, >18 years old Pediatrics and Communi- cable Diseases Objectives: Implement an evidence-based strategy for routine adult immunizations. Candia B Laughlin, RN, Key Points MS Routine immunizations for adults are: hepatitis A, hepatitis B, herpes zoster, human papilloma virus, Ambulatory Care Nursing Administration influenza, measles, mumps, rubella, meningococcal, pneumococcal, tetanus, diphtheria, pertussis and Team Members varicella. Below is a summary on priority populations, initial vaccination, and revaccination. Margie C Andreae, MD Use combination vaccines whenever possible to increase the coverage rates for vaccine-preventable Pediatrics and Communi- diseases: Tetanus-diphtheria (Td), Tetanus-diphtheria-acellular pertussis (Tdap), Measles-Mumps- cable Diseases Rubella (MMR), hepatitis A-hepatitis B (Twinrix®). Single antigen vaccines have no safety advantage. Mary K.Barry-Bodine, Live virus vaccines (Herpes Zoster, Measles-Mumps-Rubella, Varicella and Live Attenuated Influenza RN, BSN Vaccine) are contraindicated in persons who are pregnant or may become pregnant in the next four Nursing, Health Centers weeks, or who have immunocompromising conditions. If administering multiple live vaccines, give Susan G Blitz, MD, MPH simultaneously or separate them by 4 weeks. Tuberculosis (PPD) skin test should be administered General Medicine before or on the same day as a live virus vaccine or they need to be spaced 4-6 weeks apart. Katie Barwig, RN, MS This guideline follows recommendations of the federal Advisory Committee on Immunization Practices: Nursing Administration These vaccinations should be performed [strength of recommendation] for indicated populations at risk. Sherry L DeLoach, Evidence for each vaccine is based on randomized controlled trials [level of evidence] in general PharmD population and some subgroups, with findings extrapolated to some subgroups. -

(Nitags) and WHO Immunisation-Related Advisory Committees
Summary of recent issues considered by four national immunisation technical advisory groups (NITAGs) and WHO immunisation-related advisory committees Prepared by the National Centre for Immunisation Research & Surveillance (NCIRS) Period of review: 16/05/2019 to 13/09/2019 Contents 1 Advisory Committee on Immunization Practices (ACIP), USA .......................................................... 3 1.1 ACIP meeting: 26-27 June 2019 ....................................................................................................... 3 1.2 Newly published or updated recommendations ............................................................................... 20 1.2.1 Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunisation Practices.......................................................................................................................... 20 1.2.2 Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practice ..................................................................................................... 20 1.2.3 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2019–20 Influenza Season ............ 21 1.3 New or updated recommendations – not yet published ................................................................... 21 2 Immunisation Advisory Centre (IMAC), New Zealand .................................................................... -

Twinrix (Hepatitis a & B Combination)
TWINRIX (HEPATITIS A & B COMBINATION) Disease Risk & Transmission Hepatitis A (HAV) is a highly contagious liver disease with the common mode of transmission being person to person. Hepatitis A is found in the stool of infected persons and infection may be spread by contaminated water or food, by infected food handlers, after breakdown in sanitary conditions, or after floods or natural disasters, by ingestion of raw or uncooked shellfish from contaminated water, or by needle transmission (sharing needles, blood transfusions from infected persons), in daycare centers, during anal intercourse and during travel to countries in the world with poor hygienic conditions. A person who has Hepatitis A can easily spread on to others within the same household or dormitory. Symptoms include mild, flu-like symptoms, jaundice, decreased appetite, nausea and stomach pains, and diarrhea Hepatitis B (HBV) is an infection of the liver caused by the Hepatitis B virus. Hepatitis B is spread through contact with the blood of an infected person. Between 6-10 of every 100 young adults who have Hepatitis B become chronic carriers (have Hepatitis B in their blood for 6 or more months) and may be able to spread the infection to others for a long time. Hepatitis B is highly infective if there is contact with infected blood or serum through puncture wounds, splashes into the mucous membranes, contamination of open wounds, and injecting illegal drugs. It can be transmitted sexually, during birth and by sharing personal items such as razors and toothbrushes with an infected person. Symptoms include loss of appetite, tiredness, diarrhea & vomiting, pain in muscles and joints, dark urine & skin rashes. -

Hepatitis a Frequently Asked Questions
HEALTH NOTIFICATION FOR SOUTHERN NEVADA HEPATITIS A FREQUENTLY ASKED QUESTIONS WHAT ARE THE RECOMMENDATIONS is considered to be around 95 percent effective, that FOR HEPATITIS A VACCINE? protection will eventually begin to decrease and a Centers for Disease Control and Prevention (CDC) second shot boosts immunity for between 20 and 40 routinely recommends hepatitis A vaccination years, according to the CDC. for all children, for people who are at increased You can get a combination vaccine to protect you risk for infection, and for any people who want to against both hepatitis A and B. Check with your obtain immunity. health care provider. WHO SHOULD GET VACCINATED? CAN I GET MY HEPATITIS A SHOT During this current hepatitis A outbreak, the Health WHEN I GET OTHER IMMUNIZATIONS? District is working with community partners to You can receive the hepatitis A vaccine when you vaccinate people who are more likely to be infected receive other immunizations. The injection site will including people who use injection or non-injection be different. drugs (legal or illicit), people who are experiencing homelessness, and people who have recently been in IS THE HEPATITIS A VACCINE EFFECTIVE? jail or prison. Yes, the hepatitis A vaccine is highly effective in The CDC recommends hepatitis A vaccination for preventing hepatitis A virus infection. A second the following people: hepatitis A shot results in long-term protection. ¡ • All children at age of 1 year ¡ • People who are traveling to or working in countries IS THE HEPATITIS A VACCINE SAFE? where hepatitis A is common Yes, the hepatitis A vaccine is safe.