Evaluation of Antibacterial Activity of Australian
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
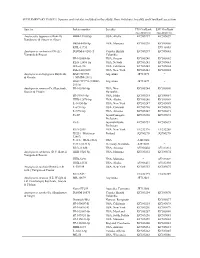
Suppl Table I
SUPPLEMENTARY TABLE I. Species and isolates included in the study, their vouchers, locality and GenBank accession Species Isolate number Locality ITS GenBank LSU GenBank accession no. accession no. Amylocystis lapponica (Romell) HHB-13400-Sp USA, Alaska KC585237 KC585059 Bondartsev & Singer ex Singer OKM-4418-Sp USA, Montana KC585238 KC585060 KHL-11755 − EU118603 Amyloporia carbonica (Overh.) DAOM-F-8281-T Canada, British KC585239 KC585061 Vampola & Pouzar Columbia FP-105585-Sp USA, Oregon KC585240 KC585062 RLG-12496-Sp USA, Nevada KC585241 KC585063 Wilcox-96 USA, California KC585242 KC585064 Zabel-40-GLN USA, New York KC585243 KC585065 Amyloporia nothofaginea Rajchenb. BAFC519794 Argentina JF713078 − & Gorjón (=MMBP-2011) BAFC519796 (=MBP- Argentina JF713079 − 2011a) Amyloporia sinuosa (Fr.) Rajchenb., FP-105386-Sp USA, New KC585244 KC585066 Gorjón & Pildain Hampshire FP-94464-Sp USA, Idaho KC585245 KC585067 HHB-12878-Sp USA, Alaska KC585246 KC585068 L-14130-Sp USA, New York KC585247 KC585069 L-6192-Sp USA, Colorado KC585248 KC585070 L-9792-Sp USA, Arizona KC585249 KC585071 Pa-3C JapanYamagata KC585250 KC585072 Prefecture Pa-3e JapanShizuoka KC585251 KC585073 Prefecture RLG-2538 USA, New York EU232196 EU232288 X725 (=Miettinen- Finland JQ700270 JQ700270 12407) P-115 (=RLG-2538) USA AJ416068 − P-211 (G-214) Germany, Karlsrube AJ345011 − RLG-1182R USA, Arizona AY966450 AY333831 Amyloporia sitchensis (D.V. Baxter) HHB-5320-Sp USA, Montana KC585252 KC585074 Vampola & Pouzar HHB-5298 USA, Montana − AY333829 HHB-12513 USA, Alaska AY966451 AY333830 -

Pecoraro, L., Perini, C., Salerni, E. & De Dominicis, V
L. Pecoraro, C. Perini, E. Salerni & V. De Dominicis Contribution to the knowledge of the mycological flora of the Pigelleto Nature Reserve, Mt. Amiata (Italy) Abstract Pecoraro, L., Perini, C., Salerni, E. & De Dominicis, V.: Contribution to the knowledge of the mycological flora of the Pigelleto Nature Reserve, Mt. Amiata (Italy). — Fl. Medit 17: 143-163. 2007. — ISSN 1120-4052. The Pigelleto Nature Reserve, situated to the south-east of Mt. Amiata (Tuscany, Italy), is char- acterized by a relict nucleus of Abies alba Mill. at low altitude, which is probably an autochtho- nous ecotype. The mycoflora list reported here is the result of past studies and observations car- ried out during 2005-2006. Among the species of macrofungi accounted for (426, belonging to 144 genera), 158 entities were collected for the first time during this recent study. Introduction This work represents a contribution to the mycological knowledge of Pigelleto Nature Reserve (Mt. Amiata, central-southern Tuscany, Italy, Fig. 1). It constitutes part of the “Life04NAT IT/000191” Project concerning the conservation of Abies alba Miller, which includes many different studies to analyze the various natural components of the area under investigation (Pecoraro & al. in press). The woods in the Amiata area are characterized by the alternation of Quercus cerris L. and Fagus sylvatica L., even though there are also mixed areas of mostly Carpinus betu- lus L. or Fraxinus sp. pl. (De Dominicis & Loppi 1992). Moreover, all of the forested areas have been subject to reforestation, mainly carried out in the first half of the 1900s due to the passage of the forestry law in 1923. -

A Phylogenetic Overview of the Antrodia Clade (Basidiomycota, Polyporales)
Mycologia, 105(6), 2013, pp. 1391–1411. DOI: 10.3852/13-051 # 2013 by The Mycological Society of America, Lawrence, KS 66044-8897 A phylogenetic overview of the antrodia clade (Basidiomycota, Polyporales) Beatriz Ortiz-Santana1 phylogenetic studies also have recognized the genera Daniel L. Lindner Amylocystis, Dacryobolus, Melanoporia, Pycnoporellus, US Forest Service, Northern Research Station, Center for Sarcoporia and Wolfiporia as part of the antrodia clade Forest Mycology Research, One Gifford Pinchot Drive, (SY Kim and Jung 2000, 2001; Binder and Hibbett Madison, Wisconsin 53726 2002; Hibbett and Binder 2002; SY Kim et al. 2003; Otto Miettinen Binder et al. 2005), while the genera Antrodia, Botanical Museum, University of Helsinki, PO Box 7, Daedalea, Fomitopsis, Laetiporus and Sparassis have 00014, Helsinki, Finland received attention in regard to species delimitation (SY Kim et al. 2001, 2003; KM Kim et al. 2005, 2007; Alfredo Justo Desjardin et al. 2004; Wang et al. 2004; Wu et al. 2004; David S. Hibbett Dai et al. 2006; Blanco-Dios et al. 2006; Chiu 2007; Clark University, Biology Department, 950 Main Street, Worcester, Massachusetts 01610 Lindner and Banik 2008; Yu et al. 2010; Banik et al. 2010, 2012; Garcia-Sandoval et al. 2011; Lindner et al. 2011; Rajchenberg et al. 2011; Zhou and Wei 2012; Abstract: Phylogenetic relationships among mem- Bernicchia et al. 2012; Spirin et al. 2012, 2013). These bers of the antrodia clade were investigated with studies also established that some of the genera are molecular data from two nuclear ribosomal DNA not monophyletic and several modifications have regions, LSU and ITS. A total of 123 species been proposed: the segregation of Antrodia s.l. -

Australia's Fungi Mapping Scheme
November 2008 AUSTRALIA’S FUNGI MAPPING SCHEME Inside this Edition: th News from the Fungimap Co-ordinator by This is our 5 Fungimap Conference and we Lee Speedy..................................................1 have organised a great programme of Contacting Fungimap ..................................2 speakers from across Australia and covering From the Editor, Instructions for authors ....3 very diverse fungi topics. In this newsletter Collating information on fungi in Australian we have included a Questionnaire, to policy & strategy documents by T May …..3 discover which Workshop topics you would Yellow/orange Amanitas by Sapphire most like to see (your Top 5 topics). Please McMullan-Fisher……………………...…..4 return this along with your Registration form Mycoacia subceracea by Barbara Paulus ....7 and we will adapt our list of Workshops New additions in W.A.'s Flora Conservation where possible. codes by Neale Bougher..............................8 New Fungimap target species....................13 At previous Conferences, transportation and Fungimap survey on Kangaroo Island by distribution of microscopes have been Paul George ..............................................13 challenging and so we have decided to add a Fungi-mapping in Ivanhoe, Melbourne by truly unique one day Masterclass with Robert Bender ..........................................15 microscopes in Sydney, to run at the UNSW, Phallus merulinus in the top end by Matt just after our Conference. This will be on the Barrett & Ben Stuckey ..............................16 topic of Disc Fungi and led by Dr. Peter Exhibition Review: In Plain View by Sarah Johnston from New Zealand. Places for this Lloyd .........................................................16 workshop will be strictly limited. Fungal News: PUBF 2008.........................17 Fungal News: SA, SEQ - QMS.................18 In early October, I accompanied Dr. -

Chapter 10 • Principles of Conserving the Arctic's Biodiversity
Chapter 10 Principles of Conserving the Arctic’s Biodiversity Lead Author Michael B. Usher Contributing Authors Terry V.Callaghan, Grant Gilchrist, Bill Heal, Glenn P.Juday, Harald Loeng, Magdalena A. K. Muir, Pål Prestrud Contents Summary . .540 10.1. Introduction . .540 10.2. Conservation of arctic ecosystems and species . .543 10.2.1. Marine environments . .544 10.2.2. Freshwater environments . .546 10.2.3. Environments north of the treeline . .548 10.2.4. Boreal forest environments . .551 10.2.5. Human-modified habitats . .554 10.2.6. Conservation of arctic species . .556 10.2.7. Incorporating traditional knowledge . .558 10.2.8. Implications for biodiversity conservation . .559 10.3. Human impacts on the biodiversity of the Arctic . .560 10.3.1. Exploitation of populations . .560 10.3.2. Management of land and water . .562 10.3.3. Pollution . .564 10.3.4. Development pressures . .566 10.4. Effects of climate change on the biodiversity of the Arctic . .567 10.4.1. Changes in distribution ranges . .568 10.4.2. Changes in the extent of arctic habitats . .570 10.4.3. Changes in the abundance of arctic species . .571 10.4.4. Changes in genetic diversity . .572 10.4.5. Effects on migratory species and their management . .574 10.4.6. Effects caused by non-native species and their management .575 10.4.7. Effects on the management of protected areas . .577 10.4.8. Conserving the Arctic’s changing biodiversity . .579 10.5. Managing biodiversity conservation in a changing environment . .579 10.5.1. Documenting the current biodiversity . .580 10.5.2. -

Piptoporus Betulinus, Fomitopsis Betulina)
Birch Fungi – Razor Strop, Birch Bracket (Piptoporus betulinus, Fomitopsis betulina) Features - This distinctive fungus only grows on birches, looks like nothing else that grows on birches, and is very common. It is not an aggressive tree killer, but is instead primarily in the business of decomposing dead trees. Birch polypore is present throughout the range of the birches, which grow around the globe in the northern hemisphere. The white-to-brownish fruiting bodies are annual, emerging from the bark of birches in spring and summer, but they deteriorate slowly and are still visible through the winter, though by then they have blackened and are not so attractive. Global Uses – Otzi the Iceman, who lived 5300 years ago, carried two fragments of a fruiting body of Fomitopsis betulina (formerly Piptoporus betulinus). Some scientists believe that Ӧtzi might have used the fungus for medical purposes and, although the idea arouses some controversy, the long tradition of the use of F. betulina in folk medicine is a fact. Infusion from F. betulina fruiting bodies was popular, especially in Russia, Baltic countries, Hungary, Romania for its nutritional and calming properties. Fungal tea was used against various cancer types, as an immunoenhancing, anti-parasitic agent, and a remedy for gastrointestinal disorders. Antiseptic and anti-bleeding dressings made from fresh F. betulina fruiting body were applied to wounds and the powder obtained from dried ones was used as a painkiller. Was used as a razor strop to sharpen fine edged blades. Medicinal Potential – Chemical Constituents: A and C, 1,3-beta-D-glucopyranan, B ergosta-7,22-dien-3- ol, fungisterol, ergosterol, agaric acid, dehydrotumulosic acid, ungalinic acid, betulinic acid, and tumulosic acid. -

Ten Principles for Conservation Translocations of Threatened Wood- Inhabiting Fungi
Ten principles for conservation translocations of threatened wood- inhabiting fungi Jenni Nordén 1, Nerea Abrego 2, Lynne Boddy 3, Claus Bässler 4,5 , Anders Dahlberg 6, Panu Halme 7,8 , Maria Hällfors 9, Sundy Maurice 10 , Audrius Menkis 6, Otto Miettinen 11 , Raisa Mäkipää 12 , Otso Ovaskainen 9,13 , Reijo Penttilä 12 , Sonja Saine 9, Tord Snäll 14 , Kaisa Junninen 15,16 1Norwegian Institute for Nature Research, Gaustadalléen 21, NO-0349 Oslo, Norway. 2Dept of Agricultural Sciences, P.O. Box 27, FI-00014 University of Helsinki, Finland. 3Cardiff School of Biosciences, Sir Martin Evans Building, Museum Avenue, Cardiff CF10 3AX, UK 4Bavarian Forest National Park, D-94481 Grafenau, Germany. 5Technical University of Munich, Chair for Terrestrial Ecology, D-85354 Freising, Germany. 6Department of Forest Mycology and Plant Pathology, Swedish University of Agricultural Sciences, P.O.Box 7026, 750 07 Uppsala, Sweden. 7Department of Biological and Environmental Science, P.O. Box 35, FI-40014 University of Jyväskylä, Finland. 8School of Resource Wisdom, P.O. Box 35, FI-40014 University of Jyväskylä, Finland. 9Organismal and Evolutionary Biology Research Programme, P.O. Box 65, FI-00014 University of Helsinki, Finland. 10 Section for Genetics and Evolutionary Biology, University of Oslo, Blindernveien 31, 0316 Oslo, Norway. 11 Finnish Museum of Natural History, P.O. Box 7, FI-00014 University of Helsinki, Finland. 12 Natural Resources Institute Finland (Luke), Latokartanonkaari 9, FI-00790 Helsinki, Finland. 13 Centre for Biodiversity Dynamics, Department of Biology, Norwegian University of Science and Technology, N-7491 Trondheim, Norway. 14 Artdatabanken, Swedish University of Agricultural Sciences, P.O. Box 7007, SE-75007 Uppsala, Sweden. -

Phd. Thesis Sana Jabeen.Pdf
ECTOMYCORRHIZAL FUNGAL COMMUNITIES ASSOCIATED WITH HIMALAYAN CEDAR FROM PAKISTAN A dissertation submitted to the University of the Punjab in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BOTANY by SANA JABEEN DEPARTMENT OF BOTANY UNIVERSITY OF THE PUNJAB LAHORE, PAKISTAN JUNE 2016 TABLE OF CONTENTS CONTENTS PAGE NO. Summary i Dedication iii Acknowledgements iv CHAPTER 1 Introduction 1 CHAPTER 2 Literature review 5 Aims and objectives 11 CHAPTER 3 Materials and methods 12 3.1. Sampling site description 12 3.2. Sampling strategy 14 3.3. Sampling of sporocarps 14 3.4. Sampling and preservation of fruit bodies 14 3.5. Morphological studies of fruit bodies 14 3.6. Sampling of morphotypes 15 3.7. Soil sampling and analysis 15 3.8. Cleaning, morphotyping and storage of ectomycorrhizae 15 3.9. Morphological studies of ectomycorrhizae 16 3.10. Molecular studies 16 3.10.1. DNA extraction 16 3.10.2. Polymerase chain reaction (PCR) 17 3.10.3. Sequence assembly and data mining 18 3.10.4. Multiple alignments and phylogenetic analysis 18 3.11. Climatic data collection 19 3.12. Statistical analysis 19 CHAPTER 4 Results 22 4.1. Characterization of above ground ectomycorrhizal fungi 22 4.2. Identification of ectomycorrhizal host 184 4.3. Characterization of non ectomycorrhizal fruit bodies 186 4.4. Characterization of saprobic fungi found from fruit bodies 188 4.5. Characterization of below ground ectomycorrhizal fungi 189 4.6. Characterization of below ground non ectomycorrhizal fungi 193 4.7. Identification of host taxa from ectomycorrhizal morphotypes 195 4.8. -

Polypore Diversity in North America with an Annotated Checklist
Mycol Progress (2016) 15:771–790 DOI 10.1007/s11557-016-1207-7 ORIGINAL ARTICLE Polypore diversity in North America with an annotated checklist Li-Wei Zhou1 & Karen K. Nakasone2 & Harold H. Burdsall Jr.2 & James Ginns3 & Josef Vlasák4 & Otto Miettinen5 & Viacheslav Spirin5 & Tuomo Niemelä 5 & Hai-Sheng Yuan1 & Shuang-Hui He6 & Bao-Kai Cui6 & Jia-Hui Xing6 & Yu-Cheng Dai6 Received: 20 May 2016 /Accepted: 9 June 2016 /Published online: 30 June 2016 # German Mycological Society and Springer-Verlag Berlin Heidelberg 2016 Abstract Profound changes to the taxonomy and classifica- 11 orders, while six other species from three genera have tion of polypores have occurred since the advent of molecular uncertain taxonomic position at the order level. Three orders, phylogenetics in the 1990s. The last major monograph of viz. Polyporales, Hymenochaetales and Russulales, accom- North American polypores was published by Gilbertson and modate most of polypore species (93.7 %) and genera Ryvarden in 1986–1987. In the intervening 30 years, new (88.8 %). We hope that this updated checklist will inspire species, new combinations, and new records of polypores future studies in the polypore mycota of North America and were reported from North America. As a result, an updated contribute to the diversity and systematics of polypores checklist of North American polypores is needed to reflect the worldwide. polypore diversity in there. We recognize 492 species of polypores from 146 genera in North America. Of these, 232 Keywords Basidiomycota . Phylogeny . Taxonomy . species are unchanged from Gilbertson and Ryvarden’smono- Wood-decaying fungus graph, and 175 species required name or authority changes. -

Polypore Fungi As a Flagship Group to Indicate Changes in Biodiversity – a Test Case from Estonia Kadri Runnel1* , Otto Miettinen2 and Asko Lõhmus1
Runnel et al. IMA Fungus (2021) 12:2 https://doi.org/10.1186/s43008-020-00050-y IMA Fungus RESEARCH Open Access Polypore fungi as a flagship group to indicate changes in biodiversity – a test case from Estonia Kadri Runnel1* , Otto Miettinen2 and Asko Lõhmus1 Abstract Polyporous fungi, a morphologically delineated group of Agaricomycetes (Basidiomycota), are considered well studied in Europe and used as model group in ecological studies and for conservation. Such broad interest, including widespread sampling and DNA based taxonomic revisions, is rapidly transforming our basic understanding of polypore diversity and natural history. We integrated over 40,000 historical and modern records of polypores in Estonia (hemiboreal Europe), revealing 227 species, and including Polyporus submelanopus and P. ulleungus as novelties for Europe. Taxonomic and conservation problems were distinguished for 13 unresolved subgroups. The estimated species pool exceeds 260 species in Estonia, including at least 20 likely undescribed species (here documented as distinct DNA lineages related to accepted species in, e.g., Ceriporia, Coltricia, Physisporinus, Sidera and Sistotrema). Four broad ecological patterns are described: (1) polypore assemblage organization in natural forests follows major soil and tree-composition gradients; (2) landscape-scale polypore diversity homogenizes due to draining of peatland forests and reduction of nemoral broad-leaved trees (wooded meadows and parks buffer the latter); (3) species having parasitic or brown-rot life-strategies are more substrate- specific; and (4) assemblage differences among woody substrates reveal habitat management priorities. Our update reveals extensive overlap of polypore biota throughout North Europe. We estimate that in Estonia, the biota experienced ca. 3–5% species turnover during the twentieth century, but exotic species remain rare and have not attained key functions in natural ecosystems. -

The Isabella Plantation Conservation Management Plan February 2012
The Isabella Plantation Conservation Management Plan February 2012 Isabella Plantation Landscape Conservation Management Plan 2012 Prepared by The Royal Parks January 2012 The Royal Parks Rangers Lodge Hyde Park London W2 2UH Tel: 020 7298 2000 Fax: 020 7402 3298 [email protected] i Isabella Plantation Conservation Management Plan CONTENTS 1.0 INTRODUCTION .............................................................................. 3 Richmond Park ............................................................................................................................................. 3 The Management Plan ................................................................................................................................ 4 Aims of the Isabella Plantation Management Plan ................................................................................ 4 Structure of the Plan .................................................................................................................................. 6 2.0 GENERAL AND MANAGEMENT CONTEXT ............................... 7 Location ......................................................................................................................................................... 7 Existing TRP Management Framework ................................................................................................ 10 Management Structure of Richmond Park .......................................................................................... 10 Landscape Management -

Universidade Federal De Santa Catarina Para a Obtenção Do Grau De Mestre Em Biologia De Fungos, Algas E Plantas
0 Gesieli Kaipper Figueiró ASPECTOS TAXONÔMICOS E FILOGENÉTICOS DE Antrodia s.l. COM A INCLUSÃO DE ESPÉCIMES DA REGIÃO NEOTROPICAL Dissertação submetida ao Programa de Pós Graduação em Biologia de Fungos, Algas e Plantas da Universidade Federal de Santa Catarina para a obtenção do Grau de mestre em Biologia de Fungos, Algas e Plantas. Orientador: Prof. Dr. Elisandro Ricardo Drechsler dos Santos. Coorientador: Dr. Gerardo Lucio Robledo. Florianópolis 2015 1 2 3 AGRADECIMENTOS Aos meus orientadores Ricardo e Gerardo, pelo aprendizado, confiança e disponibilidade durante esses dois anos, não tenho palavras para agradecê-los; Aos professores do programa de Pós-Graduação em Biologia de Fungos, Algas e Plantas pelo conhecimento transmitido durante a realização das disciplinas; Agradeço aos curadores dos Herbários CORD, FLOR, IBOT e URM, pelos empréstimos de materiais; Agradeço à Prof.ª Maria Alice pelo conhecimento transmitido e por todas as conversas e experiências no micolab; Ao Mateus Arduvino Reck pela amizade, aprendizado e disponibilidade para me ajudar sempre; Ao Dr. Aristóteles Góes Neto, que por meio do projeto “FungiBrBol” forneceu subsídios indispensáveis para a execução das análises moleculares deste trabalho; Agradeço imensamente a Val, Diogo e Carlos que tiveram toda a paciência do mundo me ensinando as coisas mais básicas e lindas sobre o mundo dos Fungos; Também sou grata aos colegas do Micolab pela amizade e carinho de sempre, compartilhando momentos de alegrias e tristezas, almoços de domingos e todas as datas comemorativas