Ecophysiology of Leucospermum R.BR.Seed Germination in Fynbos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
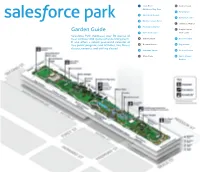
Salesforce Park Garden Guide
Start Here! D Central Lawn Children’s Play Area Garden Guide6 Palm Garden 1 Australian Garden Start Here! D Central Lawn Salesforce Park showcases7 California over Garden 50 species of Children’s Play Area 2 Mediterraneantrees and Basin over 230 species of understory plants. 6 Palm Garden -ã ¼ÜÊ ÊăØÜ ØÊèÜãE úØƀØÊèÃJapanese Maples ¼ÃØ Ê¢ 1 Australian Garden 3 Prehistoric¢ØÕ輫ÕØÊ£ØÂÜÃã«ó«ã«Üŧ¼«¹ĆãÃÜÜ Garden 7 California Garden ¼ÜÜÜŧÊÃØãÜŧÃØ¢ã«Ã£¼ÜÜÜũF Amphitheater Garden Guide 2 Mediterranean Basin 4 Wetland Garden Main Lawn E Japanese Maples Salesforce Park showcases over 50 species of 3 Prehistoric Garden trees and over 230 species of understory plants. A Oak Meadow 8 Desert Garden F Amphitheater It also offers a robust year-round calendar of 4 Wetland Garden Main Lawn free public programs and activities, like fitness B Bamboo Grove 9 Fog Garden Desert Garden classes, concerts, and crafting classes! A Oak Meadow 8 5 Redwood Forest 10 Chilean Garden B Bamboo Grove 9 Fog Garden C Main Plaza 11 South African 10 Chilean Garden Garden 5 Redwood Forest C Main Plaza 11 South African Garden 1 Children’s Australian Play Area Garden ABOUT THE GARDENS The botanist aboard the Endeavor, Sir Joseph Banks, is credited with introducing many plants from Australia to the western world, and many This 5.4 acre park has a layered soil system that plants today bear his name. balances seismic shifting, collects and filters storm- water, and irrigates the gardens. Additionally, the soil Native to eastern Australia, Grass Trees may grow build-up and dense planting help offset the urban only 3 feet in 100 years, and mature plants can be heat island effect by lowering the air temperature. -

Method to Estimate Dry-Kiln Schedules and Species Groupings: Tropical and Temperate Hardwoods
United States Department of Agriculture Method to Estimate Forest Service Forest Dry-Kiln Schedules Products Laboratory Research and Species Groupings Paper FPL–RP–548 Tropical and Temperate Hardwoods William T. Simpson Abstract Contents Dry-kiln schedules have been developed for many wood Page species. However, one problem is that many, especially tropical species, have no recommended schedule. Another Introduction................................................................1 problem in drying tropical species is the lack of a way to Estimation of Kiln Schedules.........................................1 group them when it is impractical to fill a kiln with a single Background .............................................................1 species. This report investigates the possibility of estimating kiln schedules and grouping species for drying using basic Related Research...................................................1 specific gravity as the primary variable for prediction and grouping. In this study, kiln schedules were estimated by Current Kiln Schedules ..........................................1 establishing least squares relationships between schedule Method of Schedule Estimation...................................2 parameters and basic specific gravity. These relationships were then applied to estimate schedules for 3,237 species Estimation of Initial Conditions ..............................2 from Africa, Asia and Oceana, and Latin America. Nine drying groups were established, based on intervals of specific Estimation -

PROTEACEAE – It's All About Pollination
PROTEACEAE – it’s all about pollination …….Gail Slykhuis Illustration Philippa Hesterman, images Ellinor Campbell & Marg McDonald A predominantly southern hemisphere plant family, Proteaceae is well represented in Australia, particularly in the West, but we do have our own equally special local representatives, some of which are outlined below. A characteristic feature of many genera within this plant family is the ‘pollen presenter’, which is a fascinating mechanism by which the pollen, which would otherwise be difficult to access for potential pollination vectors such as bees, birds and nectarivorous mammals, is positioned on the extended style of the flower, facilitating cross- pollination. The stigma, which is part of the style, is not mature at this time, thus avoiding self-pollination. A hand lens would enable you to clearly see pollen presenters on the following local representatives: Banksia marginata, Grevillea infecunda, Hakea spp., Isopogon ceratophyllus and Lomatia illicifolia. It is interesting to note that both Victorian Smoke-bush Conospermum mitchellii and Prickly Geebung Persoonia juniperina, also found in our district, do not have pollen presenters. Silver Banksia Banksia marginata This shrub or small tree is readily recognisable when flowering (Feb – July) by the conspicuous yellow pollen presenters, which are an obvious floral part of the banksia flower. These flowers then slowly mature into our iconic woody banksia cones. It is interesting to observe the changes in the nature of the pollen presenters as the flower develops. The white undersides of the leathery leaves provide a clue to the choice of common name with their tip being characteristically blunt or truncate. Anglesea Grevillea Grevillea infecunda One of our endemic plants, the Anglesea Grevillea was first named in 1986 and is Anglesea Grevillea found in several locations north west of Anglesea. -

Tulbagh Renosterveld Project Report
BP TULBAGH RENOSTERVELD PROJECT Introduction The Cape Floristic Region (CFR) is the smallest and richest floral kingdom of the world. In an area of approximately 90 000km² there are over 9 000 plant species found (Goldblatt & Manning 2000). The CFR is recognized as one of the 33 global biodiversity hotspots (Myers, 1990) and has recently received World Heritage Status. In 2002 the Cape Action Plan for the Environment (CAPE) programme identified the lowlands of the CFR as 100% irreplaceable, meaning that to achieve conservation targets all lowland fragments would have to be conserved and no further loss of habitat should be allowed. Renosterveld , an asteraceous shrubland that predominantly occurs in the lowland areas of the CFR, is the most threatened vegetation type in South Africa . Only five percent of this highly fragmented vegetation type still remains (Von Hase et al 2003). Most of these Renosterveld fragments occur on privately owned land making it the least represented vegetation type in the South African Protected Areas network. More importantly, because of the fragmented nature of Renosterveld it has a high proportion of plants that are threatened with extinction. The Custodians of Rare and Endangered Wildflowers (CREW) project, which works with civil society groups in the CFR to update information on threatened plants, has identified the Tulbagh valley as a high priority for conservation action. This is due to the relatively large amount of Renosterveld that remains in the valley and the high amount of plant endemism. The CAPE program has also identified areas in need of fine scale plans and the Tulbagh area falls within one of these: The Upper Breede River planning domain. -

Interim Recovery Plan No
INTERIM RECOVERY PLAN NO. 202 ALBANY WOOLLYBUSH (ADENANTHOS x CUNNINGHAMII) INTERIM RECOVERY PLAN 2005-2010 Sandra Gilfillan1, Sarah Barrett2 and Renée Hartley3 1 Conservation Officer, CALM Albany Region, 120 Albany Hwy, Albany 6330. 2 Flora Conservation Officer, CALM Albany Work Centre, 120 Albany Hwy, Albany 6330 3 Technical Officer, CALM Albany Work Centre, 120 Albany Hwy, Albany 6330 Photo: Ellen Hickman April 2005 Department of Conservation and Land Management Albany Work Centre, South Coast Region, 120 Albany Hwy, Albany WA 6331 Interim Recovery Plan for Adenanthos x cunninghammi FOREWORD Interim Recovery Plans (IRPs) are developed within the framework laid down in Department of Conservation and Land Management (CALM) Policy Statements Nos. 44 and 50. IRPs outline the recovery actions that are required to urgently address those threatening processes most affecting the ongoing survival of threatened taxa or ecological communities, and begin the recovery process. CALM is committed to ensuring that Threatened taxa are conserved through the preparation and implementation of Recovery Plans (RPs) or IRPs and by ensuring that conservation action commences as soon as possible. This IRP will operate from April 2005 to March 2010 but will remain in force until withdrawn or replaced. It is intended that, if the taxon is still ranked Endangered, this IRP will be reviewed after five years and the need further recovery actions assessed. This IRP was given regional approval on 26 October, 2005 and was approved by the Director of Nature Conservation on 26 October, 2005. The provision of funds and personnel identified in this IRP is dependent on budgetary and other constraints affecting CALM, as well as the need to address other priorities. -

(Acari: Eriophyoidea: Eriophyidae) on Leucadendron Argenteum (L.) R
Zootaxa 3085: 63–68 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) A new species of eriophyoid mite (Acari: Eriophyoidea: Eriophyidae) on Leucadendron argenteum (L.) R. Br. from South Africa DANIEL R. L. PYE The Food and Environment Research Agency, Sand Hutton, York, YO41 1LZ, United Kingdom. E-mail: [email protected] Abstract A new vagrant eriophyoid mite species, collected from plant material imported into the United Kingdom, is described and illustrated: Aceria argentae n. sp. found on Leucadendron argenteum (L.) R. Br. (Proteaceae) from South Africa. A review of the eriophyoid mite species known from plants in the Proteaceae is also provided and recent findings of non-native erio- phyoid mites in the United Kingdom are discussed. Key words: Acari, Eriophyoidea, taxonomy, key, Aceria kuko, Aculops fuchsiae Introduction The Food and Environment Research Agency (Fera) provides an identification service for plant pests and diseases for both the Department for Environment, Food and Rural Affairs (Defra) and commercial customers. This paper presents a new species of eriophyoid mite (Acari: Eriophyoidea) found on a sample intercepted by the Plant Health and Seeds Inspectorate (PHSI) and sent to Fera for examination. On 13 May 2009, a sample of Leucadendron argenteum (L.) R. Br. (Proteaceae Juss.) (silver tree, silver leaf tree, witteboom, or silwerboom) flower stalks was intercepted by Maureen Tierney (PHSI) at Heathrow Airport, Middlesex, England, from a consignment being imported into the United Kingdom from South Africa, and destined for display at the Chelsea Flower Show in England. -

Finschia-"A Genus of "Nut" Trees of the Southwest Pacific
Finschia-" A Genus of "Nut" Trees of the Southwest Pacific c. T. WHITE1 INTRODUCTION A PLANT FAMILY with a most interesting and F. Muell., Carnarvonia F. Muell., D arlin"gia F; intriguing distribution is Proteaceae, which finds Muell., Hollandaea F. Muell. (two spp.) , Mus its greatest development in Australia (650 " gravea F. Muell., and Placospermum White & species) on the one hand and South Africa (300 Francis. A surprising feature is the absence, species) on the other, though the two countries with the exception of one species in New Zea have no genera in common. Practically all the land, of the family "from Polynesia. South African species and the vast majority of There is in the islands of the southwest Paci "Australian ones are markedly xerophytic. The fic-Caroline Islands, New Guinea, Solomon largest genus, Greoillea R. Br., consists mainly Islands, and the New Hebrides-a group of trees of xerophytic shrubs or small trees but a few with the floral characters of Greuillea R. Br. are large trees found in the rain forests of and the fruit of Helicia Lour. These, I consider, tropical and subtropical eastern Australia, New all belong 'to Finschia Warb. This genus was Guinea, and New Caledonia. In the southwest founded by Warburg (1891: 297 ) on"a tree Pacific area the family finds its greatest develop from northeastern New Guinea. His original ment in northeastern Australia, where trees be description would cover Grevillea R. Br. exactly longing to it provide the great bulk of cabinet though he does not mention this genus and on timbers known in the trade as "Silky Oaks." the following page the distinctions he gives for There is close affinity between the Proteaceae of separating his proposed new genus from H elicia eastern Australia and of western South America are exactly those which distinguish Greuillea as illustrated by the genera Embothrium Forst. -

Finding Fynbos of the Western Cape, Via Grootbos
Finding Fynbos Of The Western Cape, Via Grootbos A Professional & Personal Journey To South Africa September 13th - 21st October 2018 By Victoria Ind !1 Table Of Contents 1………………………Itinerary 2………………………Introduction 3…………………….. Grootbos - My Volunteering - Green Futures Plant Nursery & Farms 4…………………….. Botanising - Grootbos Conservation Team - Hike With Sean Privett - Milkwood Forest - Self-Guided Botanising 5…………………….. Fernkloof Flower Festival 6……………………Garden Visits - Vergelegen - Lourensford - Stellenbosch - Dylan Lewis Sculpture Garden - Kirstenbosch - Green Point Diversity Garden - The Company’s Garden 7…………………… Conclusion 8…………………… Breakdown Of Expenses 9……………………. Appendix & Bibliography 10………………….. Acknowledgments !2 1: ITINERARY 13th-15th September 2018: Travel from Dublin Ireland to Cape Town. x2 nights in Cape Town. 15th September 2018: Collection from Cape Town by Grootbos Foundation, transport to Grootbos staff accommodation, Gansbaai. 16th September-15th October 2018: Volunteer work with Green Futures, a division of the Grootbos Foundation. Mainly based on the Grootbos Nature Reserve & surrounding areas of Gansbaai & Masakhane township. 20-23rd September 2018: Weekend spent in Hermanus, attend Fernkloof Flower Festival. 15th October 2018: Leave Grootbos, travel to Cape Town. 16th October 2018: Visit to Vergelegen 17th October 2018: Visit to Lourensford & Stellenbosch 18th October 2018: Visit to Dylan Lewis Sculpture Garden 19th October 2018: Visit to Kirstenbosch Botanic Garden 20th October 2018: Visit to Green Point Diversity Garden & Company Gardens 21st October 2018: Return to Dublin Ireland. Fig: (i) !3 2: INTRODUCTION When asked as a teenager what I wanted to do with my life I’d have told you I wanted to be outdoors and I wanted to travel. Unfortunately, as life is wont to do, I never quite managed the latter. -

Persoonia Levis Broad-Leaved Geebung
Persoonia levis Broad-leaved Geebung Geebung is an unusual name derived from Aboriginal languages: geebung is the name used by the Dharuk in the Sydney Region, and Jibbong by the Wiradjuri1. The genus name Persoonia, to our ears, is also unusual until you find out that it is named after a Dutch mycologist (someone who studies fungi), Christiaan Hendrik Persoon. Geebungs are endemic to Australia and there are almost 100 species which, for the most part, are found in eastern Australia, and in the SW corner of Western Australia. They are mostly small trees or shrubs. This particular species, Persoonia levis, common in Sydney bushland, grows along the central and north coast of NSW, and in the SE corner of NSW and NE corner of Victoria. We are accustomed to the subtle olives, blues, greys and yellowish greens of the foliage of the Australian bush but the Broad-leaved Geebung is quite a contrast with bright, apple green foliage. The fruits, too, are unusual, round and succulent, bright green colouring to purple, very different from the dry, hard fruits of other genera in the same (Proteaceae) family, for example, Needle Bush (Hakea), Telopea (Waratah), Grevillea and Woodly Pear (Xylomelum). Geebungs are also unusual in that they have seven chromosomes that are much larger than those of other Proteaceae2. Broad-leaved Geebung has papery bark that provides some protection from bushfires. Peel back the superficial burnt bark and you will find glorious, rich crimson beneath the blackened exterior. This species also has the potential to resprout after fires, and regenerate from seed. -

Pathogens Associated with Diseases. of Protea, Leucospermum and Leucadendron Spp
PATHOGENS ASSOCIATED WITH DISEASES. OF PROTEA, LEUCOSPERMUM AND LEUCADENDRON SPP. Lizeth Swart Thesis presented in partial fulfillment of the requirements for the degree of Master of Science in Agriculture at the University of Stellenbosch Supervisor: Prof. P. W. Crous Decem ber 1999 Stellenbosch University https://scholar.sun.ac.za DECLARATION 1, the undersigned, hereby declare that the work contained in this thesis is my own original work and has not previously in its entirety or in part been submitted at any university for a degree. SIGNATURE: DATE: Stellenbosch University https://scholar.sun.ac.za PATHOGENS ASSOCIATED WITH DISEASES OF PROTEA, LEUCOSPERMUM ANDLEUCADENDRONSPP. SUMMARY The manuscript consists of six chapters that represent research on different diseases and records of new diseases of the Proteaceae world-wide. The fungal descriptions presented in this thesis are not effectively published, and will thus be formally published elsewhere in scientific journals. Chapter one is a review that gives a detailed description of the major fungal pathogens of the genera Protea, Leucospermum and Leucadendron, as reported up to 1996. The pathogens are grouped according to the diseases they cause on roots, leaves, stems and flowers, as well as the canker causing fungi. In chapter two, several new fungi occurring on leaves of Pro tea, Leucospermum, Telopea and Brabejum collected from South Africa, Australia or New Zealand are described. The following fungi are described: Cladophialophora proteae, Coniolhyrium nitidae, Coniothyrium proteae, Coniolhyrium leucospermi,Harknessia leucospermi, Septoria prolearum and Mycosphaerella telopeae spp. nov. Furthermore, two Phylloslicla spp., telopeae and owaniana are also redecribed. The taxonomy of the Eisinoe spp. -

Protea Newsletter International
Protea Newsletter International An eNewsletter for the International Protea Industry and Scientific Community to Promote Communication, Cooperation and the Advancement of Science, Technology, Production and Marketing (and to promote the Hawaii Protea Industry) Volume 2, Number 1, April 2009 Editor: Ken Leonhardt Chairman, lnternational Protea Working Group (IPWG), International Society for Horticultural Science (ISHS) Professor, College of Tropical Agriculture and Human Resources, University of Hawaii, Honolulu, Hawaii USA Contents: A visit to South Africa ............................................................................. 2 International Horticulture Congress announcement .................................. 3 New protea poster from the University of Hawaii..................................... 4 A message from the Hawaii State Protea Growers Corporation ................ 4 A message from the Zimbabwe Protea Association .................................. 5 Protea nightlife ....................................................................................... 6 Proteaceae cultivar development and uses ................................................ 6 Sample costs to establish and produce protea ........................................... 6 Research funding awarded by the IPA...................................................... 7 New cultivar registrations......................................................................... 7 Recent books on Proteaceae .................................................................... -

Approved NSW & National Recovery Plan Eidothea Hardeniana
Approved NSW & National Recovery Plan Eidothea hardeniana September 2004 © Department of Environment and Conservation (NSW), July 2004. This work is copyright. However, material presented in this plan may be copied for personal use or published for educational purposes, providing that any extracts are fully acknowledged. Apart from this and any other use as permitted under the Copyright Act 1968, no part may be reproduced without prior written permission from NSW Department of Environment and Conservation. NSW Department of Environment and Conservation 43 Bridge Street (PO Box 1967) Hurstville NSW 2220 Tel: 02 9585 6444 www.nationalparks.nsw.gov.au Requests for information or comments regarding the recovery program for the Nightcap Oak are best directed to: The Nightcap Oak Recovery Co-ordinator Threatened Species Unit, North East Branch NSW Department of Environment and Conservation Locked Bag 914 Coffs Harbour NSW 2450 Tel: 02 6651 5946 Cover illustrator: Lesley Elkan © Botanic Gardens Trust, Sydney Cover illustration: Adult and juvenile leaves and fruit of Eidothea hardeniana This plan should be cited as follows: NSW Department of Environment and Conservation 2004, Recovery Plan for the Nightcap Oak (Eidothea hardeniana), Department of Environment and Conservation (NSW), Hurstville. ISBN 0 7313 6781 2 Recovery Plan The Nightcap Oak Recovery Plan for the Nightcap Oak (Eidothea hardeniana) Foreword The New South Wales Government established a new environment agency on 24 September 2003, the Department of Environment and Conservation (NSW), which incorporates the New South Wales National Parks and Wildlife Service. Responsibility for the preparation of Recovery Plans now rests with this new department. This document constitutes the New South Wales State and National Recovery Plan for Eidothea hardeniana Weston & Kooyman (Nightcap Oak), and as such considers the conservation requirements of the species across its range.