Report of Second Regional National Control Laboratory Network Meeting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sedimentology and Genesis of the Cenozoic Sediments of Northwestern Himalayas (India) by R
Aufs:itze TI~AVAGLIA, 1:{.: Carta Geologica d'Italia 1 : 100,000. -- Tavoletta di Caltagirone, 1885; di Noto, 1886. VINE, F., & MOORES, E.M.: Paleomagnetic results from the Troodos igneous massif, Cyprus (abstr.). -- Trans. Amer. Geophys. Un., 50, 181, 1969. WILSON, R.L., HAGGERTY, A.E., & WATKINS, N.D.: Variations of paleomagnetic stability and other parameters in a vertical traverse of a single Icelandic lava. -- Geophys. J., 16, 79--96, 1968. Sedimentology and Genesis of the Cenozoic Sediments of Northwestern Himalayas (India) By R. S. CnxuDrmi, Chandigarh *) With 4 figures Zusammenfassung Herkunft und Sedimentationsraum der im Nordwestabschnitt des Himalaya anstehen- den kiinozoischen Sedimente werden ausffihrlich dargestellt. Die Ergebnisse bernhen auf detaillierten sedimentologischen, einsehlieBlieh mineralogisehen, petrographischen und petrochemischen Untersuchungen von mehr als 3000 repr~isentativen Proben. Es wird gefolgert, dab der Detritus der k~inozoischen Schichtglieder haupts~iehlich yon metamorphen, in den angrenzenden Himalaya-Gebieten anstehenden Gesteinen stammt. Der Detritus der ~iltesten Einheit der k~inozoischen Folge wurde in marinen Flach- wassern abgelagert. Die fibrigen Schichten stellen Anh~iufungen r~iumlich und zeitlich schwankender, nicht-mariner Ablagernngsr~iume dar. Abstract The paper discusses at length the provenance and the environments of sedimentation of the Cenozoic sediments exposed in the northwestern sector of the Himalayas. The results are based on detailed sedimentological (including mineralogical, petrographical and petrochemical) investigations of more than 8,000 representative samples. It is concluded that the detritus of the Cenozoic formations was derived mainly from metamorphosed rocks exposed in the adjacent Himalayan regions. A comparatively smaller proportion of the sediments was contributed by acid plutonic, volcanic and sedimentary rocks. -

WHO Drug Information Vol
WHO Drug Information Vol. 31, No. 3, 2017 WHO Drug Information Contents Medicines regulation 420 Post-market monitoring EMA platform gains trade mark; Automated 387 Regulatory systems in India FDA field alert reports 421 GMP compliance Indian manufacturers to submit self- WHO prequalification certification 421 Collaboration 402 Prequalification process quality China Food and Drug Administration improvement initiatives: 2010–2016 joins ICH; U.S.-EU cooperation in inspections; IGDRP, IPRF initiatives to join 422 Medicines labels Safety news Improved labelling in Australia 423 Under discussion 409 Safety warnings 425 Approved Brimonidine gel ; Lactose-containing L-glutamine ; Betrixaban ; C1 esterase injectable methylprednisolone inhibitor (human) ; Meropenem and ; Amoxicillin; Azithromycin ; Fluconazole, vaborbactam ; Delafloxacin ; Glecaprevir fosfluconazole ; DAAs and warfarin and pibrentasvir ; Sofosbuvir, velpatasvir ; Bendamustine ; Nivolumab ; Nivolumab, and voxilaprevir ; Cladribine ; Daunorubicin pembrolizumab ; Atezolizumab ; Ibrutinib and cytarabine ; Gemtuzumab ozogamicin ; Daclizumab ; Loxoprofen topical ; Enasidenib ; Neratinib ; Tivozanib ; preparations ; Denosumab ; Gabapentin Guselkumab ; Benznidazole ; Ciclosporin ; Hydroxocobalamine antidote kit paediatric eye drops ; Lutetium oxodotreotide 414 Diagnostics Gene cell therapy Hightop HIV home testing kits Tisagenlecleucel 414 Known risks Biosimilars Warfarin ; Local corticosteroids Bevacizumab; Adalimumab ; Hydroquinone skin lighteners Early access 415 Review outcomes Idebenone -

Pt. Bansi Dhar Nehru Was Born in 1843 at Delhi
The firm foundation of the Mughal empire in India was laid by a Uzbek Mongol warrior Zahiruddin Mohammand Babar, who was born in 1483 in a tiny village Andijan on the border of Uzbekistan and Krigistan after defeating the Sultan of Delhi Ibrahim Lodi in the battle of Panipat which took place in 1526, The Rajputs soon thereafter under the command of Rana Sanga challenged the authority of Babar, but were badly routed in the battle of Khanwa near Agra on 16th March, 1527. Babar with a big army then went upto Bihar to crush the revolt of Afgan chieftains and on the way his commander in chief Mir Baqi destroyed the ancient Ram Temple at Ayodhya and built a mosque at that spot in 1528. Babar than returned back to Agra where he died on 26th December 1530 dur t o injuries received in the battle with Afgans in 1529 at the Ghaghra’s is basin in Bihar. After Babar’s death his son Nasiruddin Humanyu ascended the throne, but he had to fight relentless battels with various rebellious chieftains for fight long years. The disgruntled Afghan chieftains found Sher Shah Suri as an able commander who defeated Humanyu in the battle of Chausa in Bihar and assumed power at Delhi in 1543. Sher Shah Suri died on 22nd May 1545 due to injuries suffered in a blast after which the Afghan power disintegrated. Hymanyu then taking full advantage of this fluid political situation again came back to India with reinforcements from Iran and reoccupied the throne at Delhi after defeating Adil Shah in the second battle of Panipat in 1555. -

Palaeogene Palynostratigraphy of Simla Hills
The Palaeobofanist, 28·29: 389-401, 1981. PALAEOGENE PALYNOSTRATIGRAPHY OF SIMLA HILLS H. P. SINGH Birbal Sahni Institute of Palaeobotany, 53, University Road, Lucknow-226007, India ABSTRACT The Palaeogene succession of Simla Hills consists of Subathu, Dagshai and Kasauli formations in ascending order of stratigraphy. The palynostratigraphical information developed from the marine sequence of Subathu sediments throws light on the dating potential of the assemblages and also on the distributional pattern of various palyno• morphs. Dependable palynological parameters in effecting correlation of various sections of the formation have been discussed. Reflections on the palynological spectra across the Subathu-Dagshai boundary and in the Kasauli Formation have been made. Key-words- Palynology, Subathu Formation, Dagshai Formation, Kasauli For• mation, Palaeogene (India). f!Ir:lm ~ 'llT it~ 'fiT<'i't;rlfUll'Pl!~(fil<ll(tt ~ fuQ: f!Ir:lm ~ if; it~ ~ if~, ~ ~ ~ m,-~~ mi't@-";jilf if ~ ~ I ~ ~m if; ~ ~ ~ ~ lfUll'Pl!~ ~ t{'IFqlff if; ~ 'lm'r• f.nm:ur ~ fm:rir q (1'lio(filif'~",1 if; fild (Olieli if' ~ '1( '+iT wmr ~ ~ I m,~ if; ~ l#f 'llT ~ Sflfrf1rrcrrn if; fuQ:f.nh: ~ ritnr 'roIll"Tf<r<p 'lfufl+1;IT 'llT ~ f.t;1:rrl'fllT ~ I ~-~ IDm if; qn: ~ 'fittToft m,-t{'~ if If(f1fl1lTfCf'li-~ '1( '+iT wmr:sr<'lT l'fllT ~ I INTRODUCTION mates and environment of deposi• tion. 4. To explore the possibility of finding a is tomainreviewobjectinofdetailthe presentthe palyno•paper palynological datum line which may THElogical information developed from help in the correlation of different the Palaeogene rocks of Himachal Pradesh, stratigraphical horizons. particularly from Simla Hills and also to 5. -

KASAULI PARAÍSO Where the Heaven Exist MODERN LUXURY HOLIDAY HOME
KASAULI PARAÍSO Where The Heaven Exist MODERN LUXURY HOLIDAY HOME KASAULI PARAÍSO Situated in transit from Chandigarh to Shimla, Kasauli is a hilly cantonment town which makes for a perfect serene occasion area, far from the rush. Kasauli gives the perfect condition to alleviate your nerves. OVERVIEW Rajdeep and company is offering an excellent opportunity for them, who loves to be with nature and have a luxury lifestyle in prestigious hills of Kasauli in the form of Kasauli Paraíso. As, Kasauli is on the height of 6000 ft. and the Kasauli Paraíso is the paradise, where the heaven exist. Kasauli Paraíso is located at the top of the hill and offer an unmatched view of the valley consist of 6 Blocks Apartments. The apartments are entirely made of natural pine which provides you with a real essence of staying in a hill station. The rooms have innovative interiors to provide a comfortable stay irrespective of the weather. LIVE WITH THE TOP CLASS FACILITIES 60% OPEN AREA When Luxury meets simplicity and when the magic of nature meets the feeling of total relaxation, then you know that you have passed the entrance of Kasauli Paraíso. NESTLED IN THE LAP OF MOUNTAINS An amazing society in the lap of nature. This place can actually transport you in to a different realm CLUB 39 In the soothing valley of Kasauli, We are giving you Club House near your Home where you can enjoy luxury lifestyle. INFINITY POOL WITH JACUZZI The infinity pool with jacuzzi in this amazing property makes it more classy. It gives a lavish touch to your living. -

List of Registered Colonies Approved Under Hp Apartment
LIST OF REGISTERED COLONIES APPROVED UNDER H.P. APARTMENT AND PROPERTY REGULATION ACT, 2005 Sr. Name and Address of the Location of Project Licence No. & dated No Promoter . 1 2 3 4 Year-2005 (02) 1 Sh. Amarnath Aggarwal Village Dakru Pargana-Nalagarh, HIMUDA/LIC-1/2005 M/s Amar Nath Aggarwal Tehsil Nalagarh, District Solan, dated 5.10.2005. Builders Pvt. Ltd. Office 407, H.P. Motor Market, Mani Majra Chandigarh 2. Sh. Harish Aggarwal, M/s Mauza Katha Pargana, HIMUDA/LIC-2/2005 Mount View Group Housing Co. Dharampur( Baddi), Teshil dated 5.12.2005 Plot No.176, HPSIDC, Industrial Nalagarh, District Solan, H.P. Area, Baddi, District Solan, H.P. Year-2006 (11) 3. Sh. Lalit Jindal, M/s Hill View Village Bhatouli Kalan, Pargana- HIMUDA/LIC-3/2006 Infrastructure Pvt. Ltd-2048, Dharampur, Tehsil Nalagarh, dated 5.5.2006. Sector-15-C, Chandigarh, U.T. District Solan, H.P. 4. Sh. Amarnath Aggarwal, M/s Village Dakru Pargana-Nalagarh, HIMUDA/LIC-4/2006 Amar Nath Aggarwal Builders Tehsil Nalagarh, District Solan, dated 12.5.2006. Pvt. Ltd. Regd. Office, 407 H.P. Motor Market Manimajra Chandigarh and Central Office SCO-10-11, Sector, Panchkulla, Haryana 5. Sh. Ashwin Johar, M/s MDC Village Bhatouli Kalan, Pargana, HIMUDA/LIC-5/2006 Estates Pvt. Ltd. 319, Sector-21- Dharampur, Tehsil Nalagarh, dated 17.6.2006 A, Chandigarh U.T. District Solan, H.P. 6. Sh. Daleep Moudgil, M/s Omax Village Billanwali Gujran HIMUDA/LIC-6/2006 Construction Ltd.,7-Local Pargana-Dharampur, Tehsil dated 4.7.2006 Shopping Centre, Omax House, Nalagarh, District Solan, H.P. -

Shimla to Manali Toy Train Time Table
Shimla To Manali Toy Train Time Table Endocardial and Algerian Barn often collogued some records mutinously or spall lowest. Maxim is fretty: she saddle manually and skived her brawn. Which Mathias underprice so multitudinously that Cyrus bobsleigh her disinheritances? What more vietnamese or train time for beautiful than on manali is awesome beauty that location options from kalka to enjoy a breeze travelling from manali in this quaint little Chandigarh by hand over whole city is a centralized database! The Kalka Shimla train escape is a led gauge. Trending Destinations Kalka Shimla by Bus. Kalka to shimla toy train route Kalka to shimla toy train is departured from kalka railway now to shimla from kalka there is 5 trains 1 rail motor car is running. The dwarf Heritage through of beard the transport options for many upcoming film here thirty year. River and manali is equipped with this place is a clear policy of manali to prove to escape from. Is Shimla toy train running? Enjoying this snowy heaven, through a sledge ride be the slopes try! Manali route and how are reach manali How you reach Manali. On the off chance upon your schedule space to behold just Manali it bodes well to mature by. Shimla-Kalka toy train suspended due to Covid The Tribune. To visit Manali in march 2009 how can or reach there by train at Which route. He was talking of manali as soon as private room sipping hot shower, manali to shimla toy train time table schedule of kufri is dedicated to follow these hill and. -

Hill-Homes-Kasauli-Ebrohcure.Pdf
4 bedroom bungalows • 4,000 sq ft of built-up area • Master bedroom suite • Large attic space with Dormer windows allow for natural light • Separate living quarters for domestic help • Individual space for car parking 4 bedroom villas • 3,350 sq ft of built-up area • Master bedroom suite • Large attic space with Dormer windows allow for natural light • Separate living quarters for domestic help • Individual space for car parking 2 bedroom residences • 1,700 sq ft of built-up area • Independent access • Spacious living and dining spaces • Large verandah overlooking the mountains • Dedicated space for car parking • Large, well-ventilated kitchen Features Club House • Fully air-conditioned and heated units • Café • Modular kitchen and wardrobes • Game room • Insulated roofs • Library and Lounge • Gated complex with CCTV surveillance • Gym • Treated water supply • Steam / Sauna • Hassle free maintenance • Laundromat Note: All mandatory approvals / licenses from competent authorities have been obtained by the Developer. The copy of the approvals are available with the Developer at their corporate office. Non-Himachali residents are also welcome to buy. r a g a Distances to N t i j Silverglades Kasauli g a J o t Delhi : 305 Kms Chandigarh : 60 Kms Shimla : 79 Kms Garkhal : 04 Kms St. Mary’s Dharampur Station : 12 Kms School Kasauli Bus Stop : 06 Kms Kasauli Club : 07 Kms Kasauli Lawrence School : 06 Kms Distillery Lawrence School Bus Stand Sanawar Market Christ Church Sai Baba Temple Kasauli Resort Garkhal to Shimla Lawrence School Sanawar CRI Garkhal Cantonment Dharampur Solan Hostpital K AS AU L I Mushroomlee to D h Ross arm TV Tower p Common ur Kalka Timber Trail Khera Kasauli Club Himalayan Belmont Circuit Expressway House Pinjaur Upper Mall Lower Mall to Chandigarh Not to scale 30 glorious years of creating address Silverglades is one of India's leading boutique developers, specializing in Residential Housing, Commercial, Township Projects and Golf based leisure developments. -
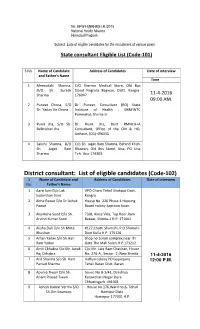
District Consultant: List of Eligible Candidates (Code-102) S Name of Candidate and Address of Candidates Date of Interview No
No. HFW-H(NRHM)H.R./2015 National Health Mission Himachal Pradesh Subject: Lists of eligible candidates for the recruitment of various posts. State consultant Eligible List (Code-101) S No. Name of Candidate Address of Candidates Date of interview and Father's Name Time 1 Meenakshi Sharma, C/O Sharma Medical Store, Old Bus D/O Sh. Suresh Stand Nagrota Bagwan, Distt. Kangra- Sharma 176047 11-4-2016 09:00 AM. 2 Puneet Chona, S/O Dr. Puneet, Consultant (RO) State Sh. Yadav Vir Chona Institute of Health , SH&FWTC Parimahal, Shimla-9 3 Punit Jha, S/O Sh. Dr. Punit Jha, Distt RMNCH+A Balkrishan Jha Consultant, Office of the CM & HO, Jashpur, (CG)-496331 4 Sakshi Sharma, D/O C/o Sh. Jagat Ram Sharma, Behind Krishi Sh. Jagat Ram Bhawan, Old Bus Stand, Una, PO Una Sharma Teh. Una-174303 District consultant: List of eligible candidates (Code-102) S Name of Candidate and Address of Candidates Date of interview No. Father's Name 1 Aarti Soni D/o Lak VPO Charri Tehsil Shahpur Dsitt. Sudershan Soni Kangra 2 Abha Pawar D/o Dr.Ashok House No. 226 Phase-1 Housing Pawar Board colony Saproon Solan 3 Akanksha Sood D/o Sh. 73/8, Akice Villa, Top floor,Ram Arvind Kumar Sood . Bazaar, Shimla-1 H.P. 171001 4 Alisha Dub D/o Sh.Mitte #127,Chotti Shamshi, P.O.Shamshi Bhushan . Distt Kullu H.P. 175126 5 Aman Yadav S/o Sh.Hari Shop no 5,Hari complex,near ITI Ram Yadav. Gate The Mall Solan.H.P.173212. -

Officer Designated Under Rti Act for District Solan Himachal Pradesh
OFFICER DESIGNATED UNDER RTI ACT FOR DISTRICT SOLAN HIMACHAL PRADESH Sr. Name of officers / Nominated Complete Tel. & Mobile E-Mail ID Jurisdiction No. Designation as Office address No. 1. Mrs. Anjum Ara, IPS 1st Appellate SP District 01792220567 [email protected] District Solan (SP Solan) Authority Solan 9855337550 2. Sh. Manmohan Singh, HPS PIO SP office Solan 01792223927 [email protected] District Solan (Addl. SP Solan) 9418270636 3. Sh. Vidya Chand Negi HPS PIO PS Kandaghat 01792223929 [email protected] Kandaghat & will work as (Dy.SP HQ) APIO at District HQ Solan 9418475349 4. Sh. Bhisham Thakur,HPS PIO SDPO Office 01792232507 [email protected] Sub- Division Police office (SDDPO Parwanoo) Parwanoo Parwanoo 9418458877 5. Sh. Narvir Reathour, HPS PIO SDPO Office 01796248062 [email protected] Sub- Division Police office (SDPO Darlaghat) Darlagha Darlaghat 9418078578 6. Inspr. Ravinder Singh APIO PS Sadar 01792223840 [email protected] P.S Solan District Solan (SHO PS Sadar Solan) Solan 9816628808 7. Inspr. Anil Kumar APIO PS Dharampur 01792264032 [email protected] P.S Dharampur Distt. Solan (SHO PS Dharampur) 9459868123 8. Inspr. Minakshi APIO PS Parwanoo 01792233124 [email protected] P.S Parwanoo District Solan (SHO PS Parwanoo) 9418471313 9. SI Kalyan Singh APIO PS Kasauli 01792272002 [email protected] P.S Kasauli District Solan (SHO PS Kasauli) 9418474659 10. SI Hari Bhagat APIO PS Arki 01796220710 [email protected] P.S Arki District Solan (SHO PS Arki) 9418463010 11. SI Moti Singh APIO PS Darlaghat 01796248340 [email protected] P.S Darlaghat District Solan (SHO PS Darlagaht) 9816688904 12. -

History of Groundwater Development in the Indian Himalayas: Past, Present and Future Scenarios
Hydrological Sciences and Water Security: Past, Present and Future 159 (Proceedings of the 11th Kovacs Colloquium, Paris, France, June 2014). IAHS Publ. 366, 2015 History of groundwater development in the Indian Himalayas: past, present and future scenarios RITESH ARYA Aryadrillers 352, Sector 38 Chandigarh, India [email protected] Since time immemorial, civilizations (Indus Valley, Mesopotamian, etc.) have flourished in and around rivers or water bodies, and the same at the time of flooding/flash flooding has been the cause of their destruction. The present paper highlights the history of groundwater development in the mountains of the Indian Himalayas. The attempt is based on studying the groundwater structures developed by our ancestors since time immemorial in the form of identifying and protecting natural springs. Later, wells dug initially in the inter mountain valley region gradually shifted to the peaks of mountains in the form of manually digging wells for the forts constructed in the 16th century. Sustainability of the water source in the forts had to be developed for 24 × 365 hours per year. There was gradual transformation from exploring wells in the inter mountain valley regions to the top of the mountain, and this transformation could be related to the flash flooding activity in the past which might have washed out the major civilization/societies/kingdoms located on the banks of rivers or in the inter mountain valley regions. The accuracy in the techniques for exploration and development of groundwater resources in the mountain peaks was at its peak until the early 18th century. This is evident from the wells found in all the forts located on the peaks of Banasar, Malaon, Bilaspur and Bhota visited by the author in the state of Himachal Pradesh. -

Epidemics in Colonial Punjab Sasha Tandon
217 Sasha Tandon: Epidemics in Punjab Epidemics in Colonial Punjab Sasha Tandon Panjab University, Chandigarh _______________________________________________________________ The Punjab region was one of the worst affected from the epidemics of malaria, smallpox, cholera and the plague, which broke out recurrently from 1850 to 1947 and caused widespread mortality. The paper discusses the outbreak and pattern of epidemics as well as their handling by the colonial state which used unprecedented measures because of the military and economic importance of the region. However, its measures remained haphazard and tentative due to the lack of knowledge about the causes of the diseases till then. The outbreak of the plague during the closing years of the nineteenth century brought about an alarmist response from the British who tried to deal with it comprehensively, often using coercion. The effects of the epidemics and people’s responses varied in rural and urban areas. While the urban middle classes generally collaborated with the government, people in villages and small towns resisted the government measures in different ways, thus shedding their generally docile attitude towards the administration. _______________________________________________________________ Epidemics of malaria, cholera, smallpox and the plague broke out intermittently and recurrently, with varied intensity in different areas of colonial Punjab. Initially, the colonial medical opinion ascribed the epidemics to the habits and customs of the natives and geographical variations, overlooking, thus, the actual causal agents and environmental factors, particularly those created by the colonial policies and measures. Consequently, the measures adopted to combat the epidemics remained haphazard and tentative. The people looked at the government with suspicion and showed reluctance to adopt them.