Connecting the Dots
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ellisbridge, Ahmedabad – 380006
S.L.U. ARTS AND H. & P. THAKORE COMMERCE COLLEGE FOR WOMEN Ellisbridge, Ahmedabad – 380006 Estd. : 1920 Managed by Gujarat Stree Kelavani Mandal RE-ACCREDITATION REPORT (CYCLE-2) Submitted to NATIONAL ASSESSMENT AND ACCREDITATION COUNCIL BANGALORE - 560072. Address: S.L.U. ARTS AND H. & P. THAKORE COMMERCE COLLEGE FOR WOMEN Nr. Ellisbridge, Ahmedabad – 380 006. Ph. 079 - 26576197 SSR of S.L.U. Arts and H. & P. Thakore Commerce College for Women 1 RE-ACCREDITATION REPORT CONTENTS Particulars Page Nos. A Preface 3 – 4 Executive Summary 5 – 7 B Profile of the Affiliated college 8 - 18 C Criteria-wise inputs 19 Criterion I Curricular Aspects 20 – 33 Criterion II Teaching-Learning and Evaluation 34 – 59 Criterion III Research, Consultancy and Extension 60 – 82 Criterion IV Infrastructure and Learning Resources 83 – 96 Criterion V Student Support and Progression 97 – 131 Criterion VI Governance, Leadership and Management 132 – 156 Criterion VII Innovation and Best Practices 157 – 162 Evaluative Reports of the Departments 163 Department of Sociology 164 – 186 Department of Home Science 187 – 203 Department of Economics 204 – 212 Department of Geography 213 – 218 Department of Commerce 219 – 229 Department of Hindi 230 – 234 Department of History 235 – 239 Department of Gujarati 240 – 245 Department of English 246 – 252 Declaration by the Head of the Institution 253 D Annexures to the Report 254 Certificates of Recognition U/s 2(f) and 12 (B) 255 – 258 Copy of accreditation certificate and peer team report of 2008 259 – 273 Quality Profile 274 Certificate of Accreditation 275 UGC Grant 12th Plan 276 – 277 Affiliation of Gujarat University 278 Certificate of Registration 279 E CD in MS Word Format - SSR of S.L.U. -

Notice of Convening the Meeting of Equity Shareholders
Sun Pharmaceutical Industries Limited Registered Office: SPARC, Tandalja, Vadodara - 390 020, Gujarat. COURT CONVENED MEETING OF SHAREHOLDERS OF SUN PHARMACEUTICAL INDUSTRIES LIMITED Day : Friday Date : January 25, 2013 Time : 10.45 a.m. Venue : Prof. Chandravadan Mehta Auditorium, General Education Centre, Maharaja Sayajirao University of Baroda, Pratapgunj, Vadodara - 390 002, Gujarat. CONTENTS PAGE NO. Notice of Court Convened Meeting of the Shareholders of Sun Pharmaceutical Industries Limited 2 Explanatory Statement under Section 393 (1) of the Companies Act, 1956 3 Scheme of Arrangement between Sun Pharmaceutical Industries Limited - Transferor Company and Sun 16 Pharma Laboratories Limited - Transferee Company and their respective members under Sections 391 to 394 of The Companies Act, 1956 Form of Proxy 29 Attendance Slip 31 1 IN THE HIGH COURT OF GUJARAT AT AHMEDABAD ORIGINAL JURISDICTION COMPANY APPLICATION NO. 373 OF 2012 In the matter of Scheme of Arrangement under Sections 391 to 394 of the Companies Act, 1956; And In the matter of Sun Pharmaceutical Industries Limited. A company incorporated under the Companies Act, 1956 and having its registered office at SPARC, Tandalja, Vadodara- 390 020, in the State of Gujarat. And Scheme of Arrangement in the nature of Spin off and Transfer of Domestic Formulation Undertaking of Sun Pharmaceutical Industries Limited to Sun Pharma Laboratories Limited Sun Pharmaceutical Industries Limited. A company incorporated under the Companies Act, 1956 and having its registered office at SPARC, Tandalja, Vadodara- 390 020, in the State of Gujarat .............................................................. Applicant Company NOTICE OF CONVENING THE MEETING OF THE EQUITY SHAREHOLDERS To, The Equity Shareholders of Sun Pharmaceutical Industries Limited. -

20172 Chhotabhai Naranbhai Patel 700 700 700
LIST OF EQUITY SHAREHOLDERS WHOSE SHARES ON WHICH DIVIDEND REMAINED UNCLAIMED / UNPAID FROM FY 2009-10 TO FY 2015-16 - TRANSFERRED TO IEPF. Folio_No/DPID Current IEPF Physical Unclaimed Transfer to Name of the Shareholder Client ID Holding Holding Suspense_Holding IEPF '00000016 TRIBHUVANDAS KISHIBHAI PATEL 1460 1460 1460 '00000036 JETHABHAI SOMABHAI PATEL 165 165 165 '00000046 GORDHANBHAI BHALABHAI PATEL 135 135 135 '00000048 KANTUBHAI CHHOTABHAI PATEL 135 135 135 '00000051 NAGINBHAI PRABHUDAS PATEL 80 80 80 '00000052 PATEL CHATURBHAI BECHARBHAI KARSANBHAI 250 250 250 '00000057 KALIDAS FULABHAI PATEL 140 140 140 '00000085 CHUNILAL MOTIBHAI PATEL 135 135 135 '00000087 MANILAL DAHYABHAI PANCHAL 80 80 80 '00000128 BHAILALBHAI MOTIBHAI PATEL 325 325 325 '00000129 DAHYABHAI JIVABHAI PATEL 655 655 655 '00000145 RAMANBHAI DAHYABHAI PATEL 165 165 165 '00000150 PARSOTTAMBHAI UMEDBHAI PATEL 135 135 135 '00000152 GOVINDBHAI KALIDAS PATEL 135 135 135 '00000160 KALIDAS LALLUBHAI PATEL 50 50 50 '00000161 SHANKERBHAI REVANDAS PATEL 270 270 270 '00000163 DHURABHAI SHANABHAI PATEL 290 290 290 '00000197 RAMANBHAI CHHOTABHAI PATEL 3545 3545 3545 '00000220 SHANTILAL ZAVERBHAI PATEL 500 500 500 '00000262 VALLAVBHAI MANSUKHBHAI PATEL 15 15 15 '00000263 FULABHAI KALIDAS PADHIAR 80 80 80 '00000271 VALIMOHAMAD GAFURBHAI VOHRA 150 150 150 '00000281 CHANCHARBEN SOMABHAI PATEL 15 15 15 '00000292 GANGABEN PARSOTTAMDAS PATEL 135 135 135 '00000299 SOMABHAI PUNJABHAI CHAVADA 140 140 140 '00000309 KANTILAL GORDHANBHAI PATEL 80 80 80 '00000325 CHHAGANBHAI VIRABHAI PARMAR -
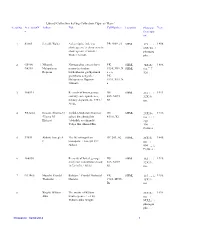
Library Collection Having Collection Type As 'Rare' Serialno Accessionn Author Title Callnumber Location Physical Year O Descripti On
Library Collection having Collection Type as 'Rare' SerialNo AccessionN Author Title CallNumber Location Physical Year o Descripti on 1 03865 Jerrold, Walter . A descriptive index to PR 2989,.J3 SHML 175 : 1996. shakespeare`s characters in 6X5cms shakespeare`s words / photogra Walter Jerrold. phs ; 2 G5900 Nilkanth, Narmgadhya athava kavi PK SHML 24X16c 1888. G6388 Mahipatram narmadashankar 1934,.N19 .N SHML ms. Rupram . lalshankarna gadhyatmak a 526 ; granthono sangrah / PK Mahipatram Rupram 1934,.N19 .N Nilkanth. a 3 166891 Records of fort st george : DS SHML 215 ; 1913. country correspondence, 485 .M272,. 32X19c military department, 1757 / R3 ms. NULL. 4 PR1254 Hosain, Shams-Ul Tarikh-I-Mubarak Shahi of DS SHML 23X15c 1931. -Ulama M yahya bin ahmad bin 459.6,.Y2 ms . Hidayat . `abdullah as-sihrindi / app. Yahya Bin Ahmad Bin. 100 : Pictures ; 5 33851 Abbott, Joseph S The life of napoleon DC 203,.A2 SHML 23X15c 1840. C . bonaparte / Joseph S C ms Abbott. 606 : Pictures ; 6 166850 Records of fort st. george : DS SHML 162 ; 1910. diary and consultation book 485 .M272,. 32X19c 1672-1678 / NULL. R3 ms. 7 G17466 Munshi, Pranlal Balidan / Pranlal Thakorlal PK SHML 135 ; 1986. Thakorlal . Munshi. 1928,.M955 . 18X12c Ba ms. 8 Wright, William The works of William 23X15c 1894. Aldis . Shakespeare / ed by ms William Aldis Wright. NULL : photogra phs ; Printed On : 02/02/2014 1 Library Collection having Collection Type as 'Rare' SerialNo AccessionN Author Title CallNumber Location Physical Year o Descripti on 41904 PRD SHML 43570 2753,.C2 SHML 43571 PRD SHML 43572 2753,.C2 SHML 43573 PRD SHML 43574 2753,.C2 SHML 43575 PRD SHML 43576 2753,.C2 SHML 43577 PRD SHML 43578 2753,.C2 SHML 43579 PRD SHML 43580 2753,.C2 SHML 43581 PRD SHML 43582 2753,.C2 SHML 43583 PRD SHML 43584 2753,.C2 SHML 43585 PRD SHML 43586 2753,.C2 SHML 43587 PRD SHML 43588 2753,.C2 SHML 43589 PRD SHML 2753,.C2 PRD 2753,.C2 PRD 2753,.C2 PRD 2753,.C2 PRD 2753,.C2 PRD 2753,.C2 PRD 2753,.C2 PRD 2753,.C2 PRD 2753,.C2 PRD 2753,.C2 PRD 2753,.C2 9 G17473 Shukla, M J . -
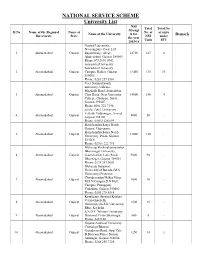
University List.Pdf
NATIONAL SERVICE SCHEME University List NSS Total Total No. Strengt Sl.No Name of the Regional Name of No. of of units Name of the University h for Remark . Directorate State NSS under the year Units SFU 2015-16 Gujarat University, Navrangpura, Near L D 1 Ahemadabad Gujarat Engineering college, 14700 147 4 Ahmedabad, Gujarat 380009 Phone:079 2630 0342 Saurashtra University, Saurashtra University 2 Ahemadabad Gujarat Campus, Rajkot, Gujarat 13450 135 33 360005 Phone: 0281 257 8501 Veer Narmad South university, Udhana - Magdalla Road, Someshwar 3 Ahemadabad Gujarat Char Rasta, Near University 14000 140 4 College, Choryasi, Surat, Gujarat 395007 Phone:0261 222 7146 Sardar Patel University, Vallabh Vidyanagar, Anand, 4 Ahemadabad Gujarat 5000 50 - Gujarat 388120 Phone: 02692 226 899 Hemchandracharya North Gujarat University, Hemchandracharya North 5 Ahemadabad Gujarat 12000 120 - University, Patan, Gujarat 384265 Phone: 02766 222 744 Maharaja Krishnakumarsinhji Bhavanagar University, 6 Ahemadabad Gujarat Gaurishanker Lake Road, 9000 90 - Bhavnagar, Gujarat 364001 Phone: 0278 243 0002 Maharaja Sayajirao University of Baroda,(M.S University) Professor Chandravadan Mehta Marg, 7 Ahemadabad Gujarat 1000 10 - M.S.N Campus,D.N Hall Campus, Pratapgunj, Vadodara, Gujarat 390002 Phone: 0265 278 8814 Krantiguru Shyamji Krishna Verma Kachchh 8 Ahemadabad Gujarat 1500 15 - University,(K.S.K University) Bhuj, Kachchh S.N.D.T Women University, 9 Ahemadabad Gujarat Diamond Circle,Bhavnagar. 800 8 - Phone 2423180 Gujarat Ayurved University, Chanakya Bhavan, -
Balraj Sahni an Autobiography
Balraj Sahni an autobiography A revealing intimate and delightful story of the life of a great actor. An insight into his life and into the world of films—the glamour, the romance, the secret lives and secret deals laid bare, as never before. A highly sensitive and brutally frank inside account of the world of the film industry. The uneasy road to stardom, the torture and the glory of success and fame.. This is Balraj Sahni by Balraj Sahni, the man adored by millions. Flash-back is an accepted technique of film-making. Unless, however, the viewers have first been made sufficiently familiar with the events that happen in the ‘present’ of the film, no flash-back is going to produce the desired effect on them. I, therefore, invite you to share same of my ‘present’ before I start unfolding before you the flash-back of my screen life. Come along then, I shall take you to a make-up room in one of the studios at Chembur. True to convention and tradition, the make-up man has applied a tilak to the mirror, before getting to work on my face. He has now finished his job. I look at myself in the mirror and notice that the ‘silver’ of my hair is showing rather prominently. Oh, yes, I have not used the khizab (dye) for several weeks now! I hastily pick, a dye pencil from the table in front of me and start vigorously drawing it across my temples. There, that’s better! While I was banishing my grey hair, the dressman called at my room to deliver my military uniform and boots, polished to perfection. -

View Annual Report
The Power of Belief Contents Message from the Chairman 2 Letter from the Managing Director & CEO 4 Board of Directors & Senior Management 6 Board Committees 6 Directors’ Report 7 Auditors’ Certificate on Corporate Governance 29 Business Overview 30 Promoting Inclusive Growth 41 Organisational Excellence 44 Management’s Discussion and Analysis 45 Key Financial Indicators 62 Particulars of Employees under Section 217 (2A) of the Companies Act, 1956 63 FINANCIALS Auditors’ Report F1 Balance Sheet F2 Profit and Loss Account F3 Cash Flow Statement F4 Schedules F5 Statement pursuant to Section 212 of the Companies Act, 1956 F44 Consolidated Financial Statements of ICICI Bank Limited and its subsidiaries F45 BASEL II – Pillar 3 Disclosures (Consolidated) F83 ENCLOSURES Notice Attendance Slip and Form of Proxy Registered Office Statutory Auditors Landmark, Race Course Circle, B S R & Co. Vadodara 390 007 Chartered Accountants, KPMG House, Kamala Mills Compound, Senapati Bapat Marg, Corporate Office Lower Parel, Mumbai 400 013 ICICI Bank Towers, Bandra-Kurla Complex, Mumbai 400 051 Registrar and Transfer Agents 3i Infotech Limited International Infotech Park, Tower 5, 3rd Floor, Vashi Railway Station Complex, Vashi, Navi Mumbai 400 703 Annual Report 2008-2009 1 Message from the Chairman As I lay down my executive responsibilities as Managing Director & CEO of ICICI Bank, I would like to thank our shareholders, the Board of Directors, government and regulatory authorities and my colleagues for their support and goodwill over the last 13 years. I am honoured to have been appointed as non-executive Chairman of the Board. In my new role, I will work with the Board and the executive management to further strengthen the governance and management The ICICI Group has always endeavoured to think framework and help the Bank to meet the ahead of the present and prepare for the future. -
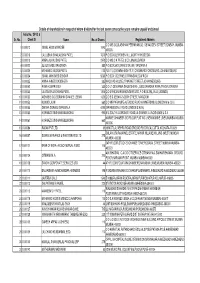
TPL List for Webiste Upload.Xlsx
Details of shareholders in respect of whom dividend for the last seven consecutive years remains unpaid/ unclaimed Folio No./ DP ID & Sr. No. Client ID Name No. of Shares Registered Address C/O M/S GOOLABKHAN PEERKHAN CO,178 NAGDEVI STREET,BOMBAY, MUMBAI- 1 0000013 ISMAIL HASSIM WADEE 1045 400003 20000016 LALLUBHAI BHAILALBHAI PATEL 7370P.O.BOX.83,BROKEN HILL,(NORTH RHODESIA) 3 0000019 AMBALAL KALIDAS PATEL 2970 C/O M/S C K PATEL & CO.,JINJA,UGANDA 4 0000022 JULIUS BASIL BRAGANZA 880 P.O.BOX.3401,DAR ES-SALAM,-TANZANIA 0 5 0000029 MOHAMED MOOSA PATEL 1100 FLAT 13,CROWN HEIGHTS,71,CROWN RD FORDSBURG,JOHANNESBURG 6 0000034 ISMAIL MAHOMED DENDAR 5060 P.O.BOX.120,ERMELO TRANSVAL,S.AFRICA 7 0000035 AMINA AHMED DADISHETH 385 KHOLVAD HOUSE,27 MARKET STREET,JOHANNESBURG 80000042 RAMA KUMARI DEVI 330 C/O LT.GEN.RAMA SHUM SHERE-,JUNG BAHADUR RANA,TANGAL DARBAR 90000045 LALITABEN JASHBHAI PATEL 1815C/O AFRICAN IRONMONGERS LTD.,P.O.BOX.286,JINJA (UGANDA) 100000052 AISHABIBI D/O EBRAHIM DAWJEE JEEWA 1320C/O E.D.JEEWA,73-28TH STREET,RANGOON 110000053 KUMUD LAJMI 660 C/O MR PARANJPE,43 TASSO ROAD HAMMERSMILK,LONDON W.6 (U.K.) 120000055 DINSHA DORABJI DARUVALA 4290209 RIBBEDALE ROAD,LONDON S.W.16, 130000060 HORMAZD DINSHAW BILLIMORIA 990813 SOUTH LA GRANGE ROAD,LA GRANGE,ILLINOIS 60525 U.S.A. MARINE CHAMBER 2ND FLOOR,FLAT NO 3,NEW MARINE LINES,MUMBAI MUMBAI- 140000061 HORMAZD DINSHAW BILLIMORIA 660 400006 150000084 RADAK PVT LTD. 55 15 MOTILAL NEHRU ROAD,GROUND FLOOR,CALCUTTA, KOLKATA-700029 GALA NO.58,RAJA INDL.ESTATE,NAHUR VILLAGE,MULUND (WEST),MUMBAI 160000097 SUNFLEX FINANCE & INVESTMENTS LTD. -

Coffee Exudes an Aura of Drama
MORPMORPMORPARIA’S PARIA’S PAGEAGEAGE E-mail: [email protected] Contents OCTOBER 2013 VOL.17/3 ○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○ THEME: Morparia’s page 2 Theatre Theatre appreciation, Mumbai ishtyle 5 V Gangadhar Managing editor English theatre is flourishing 6 Mrs. Sucharita R. Hegde Deepa Gahlot The growth of Hindi theatre 8 Editor Om Katare Anuradha Dhareshwar Theatre thrives wherever Marathi manus exists 11 Ashlesha Athavale Sub editor The vibrant hues of Gujarati theatre 14 Rajlakshmi Pillai Manvita Baradi 6 Bengali theatre and some immortal pillars 17 Design Shoma Chatterjee H. V. Shiv Shankar Theatre of Assam and Manipur: close proximity in contrast 21 Marketing Manoj Barpujari Mahesh Kanojia Know India Better Coffee - on your mind 23 OIOP Clubs Gustasp & Jeroo Irani Vaibhav Palkar Face to face: Arvind Gaur 36 Subscription National School of Drama: Time to reinvent 42 Nagesh Bangera Salim Arif In the company of theatrewalahs 44 Advisory board Quasar Thakore Padamsee 23 M V Kamath Smaller towns hungry for drama 46 Sucharita Hegde Akarsh Khurana Justice S Radhakrishnan Venkat R Chary Saga of street theatre 48 Arjun Ghosh Printed & Published by ColumnsColumnsColumns 51 Mrs. Sucharita R. Hegde for Nature watch : Bittu Sahgal One India One People Foundation, Mahalaxmi Chambers, 4th floor, In focus : C.V. Aravind 22, Bhulabhai Desai Road, Cool Champ 53 Mumbai - 400 026 Tel: 022-2353 4400 Young India 54 Fax: 022-2351 7544 36 Great Indians 56 e-mail: [email protected] / Arvind Gaur [email protected] Printed at: Graphtone (India) Pvt. Ltd. A1 /319, Shah & Nahar Industrial Estate. S. J. Marg, Lower Parel (W) Mumbai – 400 013 visit us at: Badal Sarkar Machindra Kambli Safdar Hashmi www.oneindiaonepeople.com www.facebook.com/oneindiaonepeoplefoundation oneindiaonepeople2020.blogspot.com LETTERS TO THE EDITOR You can quit if you have a firm resolve I am no regular reader of OIOP. -

Pravin Essays Essay 1
Pravin Essays Essay 1 Vachanamrut, a Unique Scripture: The Divine Experience of Fulfilment of life In the history of this spiritual world, the Vachanamrut stands on as an only unique scripture. The Vachanamrut’s dialects are the essence of experiences and the difficult spiritual path is made easier to follow. This fills up the gap of emptiness in life. It helps the soul’s incompleteness accomplish its desired goal and helps the soul experience its divinity and fulfilment. The Vachanamrut or the nectarine discourses of Bhagwan Swaminarayan is the most sacred and foundational scripture of the Swaminarayan Sampradaya. It contains the profound wisdom of the Vedas, Upanishads, Brahma sutras, Bhagwad Gita, Bhagavat, Purana, Dharma shastras like Yagnavalka Smruti, Vidurniti, and epics like the Ramayana and Mahabharat. It is the revealed fact, that the words were spoken by the supreme Reality Himself, Bhagwan Swaminarayan who had mastered the scriptures and Ashtang-Yoga and also scrutinized the beliefs and practices of people throughout India. In fact, in Vach. Gad II 28 – Shriji Maharaj has gone so far and said, “I have delivered this discourse having heard and having extracted the essence from the Vedas, the Shastras. The Puranas and all other words of this earth pertaining to liberation. This is the most profound fundamental principle; it is the essence of all essences.” It is the essence of ancient Indian wisdom given by Bhagwan Swaminarayan and complied by four contemporary scholarly-sadhus who were known for their asceticism and scholarship in Sanskrit, besides their devotion to Him. It was even reviewed and approved by Bhagwan Swaminarayan during its compilation, a fact is evident in Vach. -

SPIL Proxy Form & Attendance Slip
Registered Office : SPARC, Tandalja, Vadodara - 390 020. Registered Office : SPARC, Tandalja, Vadodara - 390 020. Corporate Office: Acme Plaza, Andheri-Kurla Road, Andheri (East), Mumbai - 400 059. Corporate Office: Acme Plaza, Andheri-Kurla Road, Andheri (East), Mumbai - 400 059. ATTENDANCE SLIP ATTENDANCE SLIP NINETEENTH ANNUAL GENERAL MEETING, Friday, September 16, 2011, at 10.30 a.m. NINETEENTH ANNUAL GENERAL MEETING, Friday, September 16, 2011, at 10.30 a.m. Sr. No. : Sr. No. : Name: Name: Address: Address: Folio/D.P & Client I.D. No. : Folio/D.P & Client I.D. No. : No. of Shares held : No. of Shares held : I/We hereby record my presence at the NINETEENTH ANNUAL GENERAL MEETING of the Company held on Friday, I/We hereby record my presence at the NINETEENTH ANNUAL GENERAL MEETING of the Company held on Friday, September 16, 2011, at 10.30 a.m. at Prof. Chandravadan Mehta Auditorium, General Education Centre, Maharaja Sayajirao September 16, 2011, at 10.30 a.m. at Prof. Chandravadan Mehta Auditorium, General Education Centre, Maharaja Sayajirao University of Baroda, Pratapgunj, Vadodara - 390 002, Gujarat. University of Baroda, Pratapgunj, Vadodara - 390 002, Gujarat. SIGNATURE OF THE ATTENDING MEMBER/PROXY___________________________________________________________ SIGNATURE OF THE ATTENDING MEMBER/PROXY___________________________________________________________ Notes: 1. Shareholder/Proxyholder wishing to attend the meeting must bring the Attendance Slip to the meeting and hand over at Notes: 1. Shareholder/Proxyholder wishing to attend the meeting must bring the Attendance Slip to the meeting and hand over at the entrance duly signed. the entrance duly signed. 2. Shareholder/Proxyholder desiring to attend the meeting should bring his/her copy of the Notice for reference at the 2. -
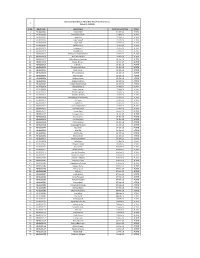
I Sr No Agent Code Agent Name Commencement Date Status 1
Advisor On-Boarded List Aditya Birla Health Insurance Co Ltd.