Nota Lepidopterologica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental and Social Impact Assessment Seismic Reflection Survey and Well Drilling, Umkhanyakude District Municipality, Northern Kzn
SFG1897 v2 Public Disclosure Authorized ENVIRONMENTAL AND SOCIAL IMPACT ASSESSMENT SEISMIC REFLECTION SURVEY AND WELL DRILLING, UMKHANYAKUDE DISTRICT MUNICIPALITY, NORTHERN KZN Public Disclosure Authorized Client: SANEDI–SACCCS Consultant: G.A. Botha (PhD, Pr.Sci.Nat) in association with specialist consultants; Brousse-James and Associates, WetRest, Jeffares & Green, S. Allan Council for Geoscience, P.O. Box 900, Pietermaritzburg, 3200 Council for Geoscience report: 2016-0009 June, 2016 Copyright © Council for Geoscience, 2016 Public Disclosure Authorized Public Disclosure Authorized Table of Contents Executive Summary ..................................................................................................................................... vii 1 Introduction ........................................................................................................................................... 1 2 Project description ................................................................................................................................ 4 2.1 Location and regional context ....................................................................................................... 5 2.2 2D seismic reflection survey and well drilling; project description and technical aspects ............ 7 2.2.1 Seismic survey (vibroseis) process ....................................................................................... 7 2.2.2 Well drilling ........................................................................................................................... -

Redalyc.On the Recent Invasion of the Canary Islands by Two Butterfly
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Wiemers, M.; Acosta-Fernández, B.; Larsen, T. B. On the recent invasion of the Canary Islands by two butterfly species, with the first record of Leptotes pirithous (Linnaeus, 1767) from Gran Canaria, Spain (Lepidoptera: Lycaenidae) SHILAP Revista de Lepidopterología, vol. 41, núm. 161, marzo, 2013, pp. 95-104 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45528755006 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 95-104 On the recent invasion o 10/3/13 18:45 Página 95 SHILAP Revta. lepid., 41 (161), marzo 2013: 95-104 CODEN: SRLPEF ISSN: 0300-5267 On the recent invasion of the Canary Islands by two butterfly species, with the first record of Leptotes pirithous (Linnaeus, 1767) from Gran Canaria, Spain (Lepidoptera: Lycaenidae) M. Wiemers, B. Acosta-Fernández & T. B. Larsen Summary Leptotes pirithous is reported from Gran Canaria for the first time, the recent spreading of this species and of Cacyreus marshalli in the Macaronesian Islands is discussed, and distribution maps of both species are presented for the Canary Islands. KEY WORDS: Lepidoptera, Lycaenidae, Leptotes pirithous, Gran Canaria, Spain. Sobre la reciente invasion de dos mariposas en las Islas Canarias con el primer registro de Leptotes pirithous (Linnaeus, 1767) de Gran Canaria, España (Lepidoptera: Lycaenidae) Resumen Se registra por primera vez para Gran Canaria a Leptotes pirithous, se discute la reciente expansión de esta especie y de Cacyreus marshalli en Macaronesia y se dan mapas de distribución de ambas especies presentes en las Islas Canarias. -

Butterflies and Skippers of the South East Aegean Island of Hálki, Dhodhekánisa (= Dodecanese) Island Complex, Greece, Representing 16 First Records for the Island
Butterflies and Skippers of the South East Aegean Island of Hálki, Dhodhekánisa (= Dodecanese) Island Complex, Greece, representing 16 first records for the island. First record of Cacyreus marshalli from the Greek Island of Sími. An update of the Butterfly and Skipper Fauna of the Greek Island of Rhodos (Lepidoptera: Papilionoidea & Hesperioidea) Christos J. Galanos Abstract. Sixteen records of butterflies and skippers from Hálki Island are now being presented for the first time ever. Cacyreus marshalli is being reported as new to Sími Island, and an update of the butterflies and skippers of Rhodos Island is being given together with a comparative study of the butterfly and skipper fauna for the lepidopterologically better known islands of the Dhodhekánisa-group. Samenvatting. De waarnemingen van 16 soorten dagvlinders en dikkopjes van het Griekse eiland Hálki (Dodekanesos) worden voor het eerst vermeld. Cacyreus marshalli wordt voor het eerst vermeld van het eiland Sími en de dagvlinderfauna van het eiland Ródos wordt bijgewerkt gevolgd door een vergelijkende studie van de beter bestudeerde eilanden van de Dodekanesos eilandengroep. Résumé. Seize espèces de papillons de jour sont mentionnées ici pour la première fois de l’île grecque de Hálki (Dodecanèse). Cacyreus marshalli est mentionné pour la première fois de l’île de Sími. La faune lépidopoptérologique de l’île de Rhodes est mise à jour, suivie par une étude des papillons des îles du Dodécanèse mieux étudiées en ce qui concerne les papillons. Κey words: Greece – Dhodhekánisa (= Dodecanese) Islands – Hálki Island – Sími Island – Rhodos Island – Lepidoptera – Papilionoidea – Hesperioidea – Cacyreus marshalli – Faunistics. Galanos C. J.: Parodos Filerimou, GR-85101 Rodos (Ialisos), Greece. -
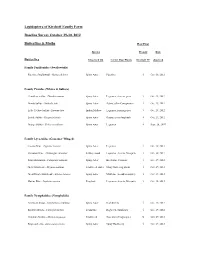
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

FINAL REPORT Biodiversity Assessment, and Strategic
FINAL REPORT Biodiversity assessment, and strategic conservation planning, for the riparian forests of the Spiny Forest Ecoregion of Madagascar. By: Alison Cameron (PhD student, Leeds University, UK) and Maminarina Dutel Ravoninjatovo (DEA student, University of Tulear, Madagascar) Introduction Madagascar has been called the highest biodiversity priority in the world but biodiversity conservation priorities within Madagascar are only now being identified. Recent research, biodiversity planning and conservation action have focused on east coast rainforest areas, while other areas of potentially very high biological interest have largely been ignored. The WWF Spiney Forests Ecoregion action plan indicates that West coast dry forests and riparian forests are under-represented in Madagascar’s reserve system, and are being degraded and fragmented at very high rates. This project is therefore addressing deficiencies in biodiversity data for this region in collaboration with The University of Tulear, WWF, and Frontier. Project Aims and Objectives To provide an opportunity for transfer of entomological survey skills from Alison Cameron (PhD student) to a Malagasy DEA student (Maminarina Dutel Ravoninjatovo). To provide the DEA student with experience of project decision making processes and meeting project by providing opportunities to integrate with project teams from WWF (World Wide Fund For Nature) and Frontier Madagascar. The production of a DEA thesis, co-supervised by the University of Tulear and WWF staff in Tulear. To make field survey data publically available through the national biodiversity data base, known as the “Platforme D’Analyse” (PDA)*. Training Initial training was conducted in Beza Mahafaly Special Reserve, between the 29th - 31st Oct 2002. Alison spent 3 days in the field training Dutel (Photo 1 right) and also Edidy (Photo 1, left) who runs the small natural history museum at Beza Mahafaly. -

Contribution to the Knowledge of Lepidoptera Fauna of Lampedusa
Journal Journal of Entomological of Entomological and and Acarological Acarological Research Research 2019; 2012; volume volume 51:8031 44:e ENTOMOLOGY Contribution to the knowledge of Lepidoptera fauna of Lampedusa: Bifascioides leucomelanella (Rebel, 1917) and Ceutholopha isidis (Zeller, 1867) (Lepidoptera) new to Italy M. Pinzari,1 M. Pinzari2 1Department of Biology, University of Roma Tor Vergata, Rome: 2Amateur entomologist, Rome, Italy (Pinzari, 2016b; Pinzari & Pinzari, 2019a,b; Pinzari et al., Abstract 2018b) and biology (Pinzari & Sbordoni, 2013; Pinzari, 2016a, 2019; Pinzari et al., 2017, 2018a, 2019, 2019a) of Lepidoptera in For the first time, 13 species are reported for the Lepidoptera Italy are still scarcely known. Recently, the survey has also been fauna of Lampedusa Island. Bifascioides leucomelanella and extended to the southern Italy and isles leading results that Ceutolopha isidis are new to Italy. The presence of Azanus ubaldus prompt further investigation. is confirmed. During a short surveyonly on Lepidoptera fauna in Lampedusa we collected a few species that are still unknown for the island. Although short surveys lead to the collection of few specimens Introduction and species, however they can reveal interesting species of bio- geographicuse relevance (e.g., Acleris lorquiniana (Duponchel, 1835), rare species in Italy, Pinzari & Pinzari, 2013; Scythris Research on Lepidoptera fauna in Central Italy has been car- clavella (Zeller, 1855), new to Peninsular Italy, Pinzari, 2016; ried out for many years and showed how much the fauna Clepsis peritana (Clemens, 1860), an alien species, Pinzari et al., 2018) and give an important contribution in shaping their distribu- tion in Italy. Correspondence: Manuela Pinzari, Department of Biology, University In this framework, we report the species newly recorded for Tor Vergata of Rome, via della Ricerca Scientifica 1, 00133 Rome, Italy. -

Specimen Records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895
Catalog: Oregon State Arthropod Collection 2019 Vol 3(2) Specimen records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895 Jon H. Shepard Paul C. Hammond Christopher J. Marshall Oregon State Arthropod Collection, Department of Integrative Biology, Oregon State University, Corvallis OR 97331 Cite this work, including the attached dataset, as: Shepard, J. S, P. C. Hammond, C. J. Marshall. 2019. Specimen records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895. Catalog: Oregon State Arthropod Collection 3(2). (beta version). http://dx.doi.org/10.5399/osu/cat_osac.3.2.4594 Introduction These records were generated using funds from the LepNet project (Seltmann) - a national effort to create digital records for North American Lepidoptera. The dataset published herein contains the label data for all North American specimens of Lycaenidae and Riodinidae residing at the Oregon State Arthropod Collection as of March 2019. A beta version of these data records will be made available on the OSAC server (http://osac.oregonstate.edu/IPT) at the time of this publication. The beta version will be replaced in the near future with an official release (version 1.0), which will be archived as a supplemental file to this paper. Methods Basic digitization protocols and metadata standards can be found in (Shepard et al. 2018). Identifications were confirmed by Jon Shepard and Paul Hammond prior to digitization. Nomenclature follows that of (Pelham 2008). Results The holdings in these two families are extensive. Combined, they make up 25,743 specimens (24,598 Lycanidae and 1145 Riodinidae). -

Pollinator Availability: a Possible Explanation of in Inter.Island Floral Variation "Iuszcia Gai-Apagan a (A Cantha Ceae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aquatic Commons 22 NOTICIAS DE GALAPAGOS No.54 POLLINATOR AVAILABILITY: A POSSIBLE EXPLANATION OF IN INTER.ISLAND FLORAL VARIATION "IUSZCIA GAI-APAGAN A (A CANTHA CEAE) By: Conley K. McMullen INTRODUCTION ducted, resulting in 53 angiospenns being classified as self-compatible, \^/ith 50 of these capable of auto- Floristic studies in Galápagos have had an admi- gamy (Aide, 1986; Elisens, 1989; McMullen, 1990; rable history, and have culminated in works such as Rick, 1966). Only one species has been classified as 'Flora of the Galápagos Islands' (V/iggins and Por- self-incompatible (Grant and Grant, 1981). ter, l97I) and 'An updated and annotated check list Based on the results of these breeding studies and of the vascular plants of the Galápagos Islands' the fact that there are relatively few potential pollina- (Lawesson et al., l98l ). However, an understanding tors in the Galápagos, it has been hypothesized that of the relationships that exist between the members the first angiosperms to colonize the islands were of this unique flora and their insect coinhabitants is those that possessed upon arrival, or developed soon still in its infancy. after, the ability to reproduce autogamously (Aide, Stewart (1911), after visiting the Galápagos Is- 1986; McMullen, 1981,1990; Rick, 1966). The ini- lands as part of the California Academy of Sciences tial scarcity of pollinating insects, including X. expedition in 1905-6, made one of the earliest refer- darwini, could also explain the small flower size and ences to this subject when he noted that most of the drab color of the maj ority of endemics. -

The Status and Distribution of Mediterranean Butterflies
About IUCN IUCN is a membership Union composed of both government and civil society organisations. It harnesses the experience, resources and reach of its 1,300 Member organisations and the input of some 15,000 experts. IUCN is the global authority on the status of the natural world and the measures needed to safeguard it. www.iucn.org https://twitter.com/IUCN/ IUCN – The Species Survival Commission The Species Survival Commission (SSC) is the largest of IUCN’s six volunteer commissions with a global membership of more than 10,000 experts. SSC advises IUCN and its members on the wide range of technical and scientific aspects of species conservation and is dedicated to securing a future for biodiversity. SSC has significant input into the international agreements dealing with biodiversity conservation. http://www.iucn.org/theme/species/about/species-survival-commission-ssc IUCN – Global Species Programme The IUCN Species Programme supports the activities of the IUCN Species Survival Commission and individual Specialist Groups, as well as implementing global species conservation initiatives. It is an integral part of the IUCN Secretariat and is managed from IUCN’s international headquarters in Gland, Switzerland. The Species Programme includes a number of technical units covering Species Trade and Use, the IUCN Red List Unit, Freshwater Biodiversity Unit (all located in Cambridge, UK), the Global Biodiversity Assessment Initiative (located in Washington DC, USA), and the Marine Biodiversity Unit (located in Norfolk, Virginia, USA). www.iucn.org/species IUCN – Centre for Mediterranean Cooperation The Centre was opened in October 2001 with the core support of the Spanish Ministry of Agriculture, Fisheries and Environment, the regional Government of Junta de Andalucía and the Spanish Agency for International Development Cooperation (AECID). -

Insect Egg Size and Shape Evolve with Ecology but Not Developmental Rate Samuel H
ARTICLE https://doi.org/10.1038/s41586-019-1302-4 Insect egg size and shape evolve with ecology but not developmental rate Samuel H. Church1,4*, Seth Donoughe1,3,4, Bruno A. S. de Medeiros1 & Cassandra G. Extavour1,2* Over the course of evolution, organism size has diversified markedly. Changes in size are thought to have occurred because of developmental, morphological and/or ecological pressures. To perform phylogenetic tests of the potential effects of these pressures, here we generated a dataset of more than ten thousand descriptions of insect eggs, and combined these with genetic and life-history datasets. We show that, across eight orders of magnitude of variation in egg volume, the relationship between size and shape itself evolves, such that previously predicted global patterns of scaling do not adequately explain the diversity in egg shapes. We show that egg size is not correlated with developmental rate and that, for many insects, egg size is not correlated with adult body size. Instead, we find that the evolution of parasitoidism and aquatic oviposition help to explain the diversification in the size and shape of insect eggs. Our study suggests that where eggs are laid, rather than universal allometric constants, underlies the evolution of insect egg size and shape. Size is a fundamental factor in many biological processes. The size of an 526 families and every currently described extant hexapod order24 organism may affect interactions both with other organisms and with (Fig. 1a and Supplementary Fig. 1). We combined this dataset with the environment1,2, it scales with features of morphology and physi- backbone hexapod phylogenies25,26 that we enriched to include taxa ology3, and larger animals often have higher fitness4. -

Diversity and Ecology of Butterflies and Moths in Wadi Gaza, Gaza Strip, Palestine
International Journal of Scientific and Research Publications, Volume 5, Issue 11, November 2015 707 ISSN 2250-3153 Diversity and Ecology of Butterflies and Moths in Wadi Gaza, Gaza strip, Palestine Zuhair .W. Dardona*, Ayman .W. Dardona**, Mohammed.A.Albayoumi * * Msc Microbiology ** Msc Limnology Abstract- Butterflies and moths were studied in regions of Wadi Gaza, extending from Salahe El-deen bridge west to Wadi Abo- Qatron near Wadi Gaza village to the east. The research is based on studying the diversity of butterflies and moths in terms of taxa diversity, Genera compositions, and family abundance. In terms of family abundance, the survey showed that all recorded butterflies are belonging to five main families (Pieridae, Hesperiidae, Lycaenidae, Nymphalidae, Papilionidae). The recorded moths are also belonging to five families (Arctiidae, Crambidae, Geometridae, Noctuidae, Sphingidae). In terms of species and genera compositions and diversity, the survey revealed that butterflies are more abundant concerning diversity and richness than moths. The five families of butterflies are consisting of (19 genera) and (25 species) while the five families of moths are consisting of only (10 genera) and (11species).The butterflies represented (69 %) of recorded species in the area of study while the moths were represented in (31 %) of the findings. The most abundant family of butterflies is Pieridae with (36%) of all recorded butterflies, followed by Lycaenidae (32%). As for moths, the abundant families are Noctuidae, Geometridae, and Crambidae were each family was represented by (3 species), and they form (82%) of recorded moths. In this study all genera, in both moths and butterflies are represented only by one specie except six genera of butterflies and one genus of moths as each one is represented with two species, these six genera of butterflies are zizeeria,Vanessa, Colias, Pieris, Carcharodus, and Pointa and that genues of moths is Stemorrhages. -

South Africa's Drakensberg Mountains & Zululand
CRANE'S CAPE TOURS & TRAVEL P.O.BOX 26277 * HOUT BAY * 7872 CAPE TOWN * SOUTH AFRICA TEL / FAX: (021) 790 5669 CELL: 083 65 99 777 E-Mail: [email protected] Drakensberg Mountains and Zululand 26 January – 10 February 2017 Holiday participants John and Jan Croft Malcolm and Helen Crowder Peter and Monica Douch Barbara Wheeler Helen Young David and Barbara Lovell John Coish Jean Dunn Chris Durdin and John Durdin Leaders: Geoff Crane and Bruce Terlien www.naturalhistorytours.co.za Holiday report by Chris Durdin. All the photos in this report were taken during the holiday by group members. Cover: top row – red bishop and elephant parade at Hluhluwe-Imfolozi Game Park (JCr). Middle row – Common diadem ♂ (JCr); vervet monkey and butterfly lobelia (BL). Bottom row – Black-bellied starling and male impalas (JCr). More photos from the holiday are via www.honeyguide.co.uk/wildlife-holidays/drakenbergandzululand.html We stayed at Drakensbergs: Mont Aux Sources hotel www.montauxsources.co.za Bonamanzi Game Reserve www.bonamanzi.co.uk Wakkerstroom: Wetlands Guest House www.wetlandscountryhouse.co.za and De Kotzenhof Guest House www.dekotzenhof.co.uk The group in Hluhluwe-Imfolozi Game Park, with elephants in the background. Peter and Monica were elsewhere when the photo was taken by Geoff, so he is also missing. This holiday, as for every Honeyguide holiday, also puts something into conservation in our host country by way of a contribution to the wildlife that we enjoyed. The conservation contributions this year of £40 per person were supplemented by gift aid through the Honeyguide Wildlife Charitable Trust giving a total of £630, a little over 10,250 rands, sent to the second Southern African Bird Atlas Project (SABAP2), an intensive monitoring programme undertaken in South Africa and adjacent countries.