Supporting Information For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MEMBERSHIP DIRECTORY Australia University of Guelph International Psychoanalytic U
MEMBERSHIP DIRECTORY Australia University of Guelph International Psychoanalytic U. Berlin University College Cork Curtin University University of LethbridGe Justus Liebig University Giessen University College Dublin La Trobe University University of Ottawa Karlsruhe Institute of TechnoloGy University of Ulster Monash University University of Toronto Katholische Universität Eichstätt- Italy National Tertiary Education Union* University of Victoria Ingolstadt SAR Italy Section University of Canberra Vancouver Island University Leibniz Universität Hannover European University Institute University of Melbourne Western University Mannheim University of Applied International School for Advanced University of New South Wales York University Sciences Studies (SISSA) University of the Sunshine Coast Chile Max Planck Society* International Telematic University Austria University of Chile Paderborn University (UNINETTUNO) Ruhr University Bochum Magna Charta Observatory Alpen-Adria-Universität Klagenfurt Czech Republic RWTH Aachen University Sapienza University of Rome MCI Management Center Innsbruck- Charles University in Prague Technische Universität Berlin Scuola IMT Alti Studi Lucca The Entrepreneurial School Palacký University Olomouc University of Graz Technische Universität Darmstadt Scuola Normale Superiore Vienna University of Economics and Denmark Technische Universität Dresden Scuola Superiore di Sant’Anna Business SAR Denmark Section Technische Universität München Scuola Superiore di Catania University of Vienna Aalborg University TH -

Workshop at the Chair of Modern History: “Travelogues of the Orient in the Nineteenth and Early Twentieth Centuries” 27 November 2020 Online Platform: Cisco Webex
Illustrated by Luigi Mayer (1755-1803), Rawpixel/ image distorted in length/* Workshop at the Chair of Modern History: “Travelogues of the Orient in the Nineteenth and Early Twentieth Centuries” 27 November 2020 Online Platform: Cisco Webex Chair: Prof. Dr. Carola Dietze Organiser: Uğur Özcan, PhD Research Assistants: Lisa Gersdorf (Organization and scheduling), Sebastian Hansen (Layout) Supported by Academics in Solidarity, Free University Berlin & History Institute of Friedrich Schiller University Jena Email: [email protected] * CCBY-2.0, https://creativecommons.org/licenses/by/2.0/deed.en; https://www.flickr.com/photos/vintage_illustration/42077001034/in/photostream/ CONTENTS Introduction about the Workshop ...................................................................................... 2 Poster .................................................................................................................................... 3 Speakers and Commentators ............................................................................................... 4 Information .......................................................................................................................... 4 Abstracts of the Workshop .................................................................................................. 5 Participants ..........................................................................................................................9 1 Workshop at the Chair of Modern History: “Travelogues of the Orient in the Nineteenth -

List of Participants
List of participants Alonso-Ruiz, Patricia, Ulm University, Germany [email protected] Ayache, Antoine, Lille University of Science and Technology, France [email protected] Baker, Simon, University of Manchester, United Kingdom [email protected] Bandt, Christoph, University of Greifswald, Germany [email protected] Bara´nski,Krzysztof, University of Warsaw, Poland [email protected] Barany, Balazs, Budapest University of Technology and Economics, Hungary [email protected] Barnsley, Michael, Australian National University, Australia [email protected] Barral, Julien, University of Paris 13, France [email protected] Berlinkov, Artemii, -, Russia [email protected] Bohl, Tilman, University of Jena, Germany [email protected] Boutard, Geoffrey, Lille University of Science and Technology, France [email protected] Buczolich, Zoltan, E¨otv¨osLor´andUniversity, Hungary [email protected] B¨ohm,Markus, University of Jena, Germany [email protected] Capitanelli, Raffaela, Sapienza University of Rome, Italy [email protected] Charmoy, Philippe, University of Oxford, United Kingdom [email protected] Chen, Changhao, University of Oulu, Finland [email protected] Chen, Joe, University of Connecticut, United States [email protected] Chen, Zhen-Qing, University of Washington, United States [email protected] Deli`ege,Adrien, University of Li`ege,Belgium 1 [email protected] Don, Henk, Radboud University Nijmegen, Netherlands [email protected] -

CELS Accepted Papers Program Committee 2016-11-04
Friday, November 18, 2016 8:00-11:00 AM Breakfast/Registration (3rd Floor Loggia) 9:00-11:00 AM Methods Panel (Room 3041) 11:00-11:15 AM Break (3rd and 4th Floor Loggias) Panel 1 Room TBA Room TBA Room TBA Room TBA Room TBA Room TBA Room TBA International/ Comparative Relational and Incomplete Accounting & Law II Administrative Law Cooperation Law Law & Psychology I Contracts I Securities Law II Paper: Does Counter-Cyclical Paper: An Autopsy of Paper: Estimating the Compliance Paper: The Legalization of Paper: Barriers to Contracting in Provisioning Mitigate Lending Paper: Can Nudges Be Cooperation: Diamond Dealers Paper: N-Equality: More People, Costs of Securities Regulation: A Truth in International Fact- Village Economies: A Test for Contractions? Evidence from Supply Transparent and Yet Effective? and the Limits of Trust-Based Less (Concern for) Equality? Bunching Analysis of Sarbanes- Finding Enforcement Constraints Shocks Exchange Oxley Section 404(B) Author: Hendrik Bruns University of HamBurg - School of Business, Economics and Social Sciences; Max Planck Society for the Author: Sudarshan Author: Ryan Bubb New York Advancement of the Sciences - Author:Stephen M. Garcia JayaramanUniversity of Rochester - Author: Shiri Krebs Stanford University School of Law Supreet International Max Planck University of Michigan Simon Business School Bryce University, School of Law, Kaur ColumBia University Sendhil Research School on Earth System Author: Barak D. Richman Duke Avishalom Tor Notre Dame Law Author: Dhammika Dharmapala Schonberger -

Curriculum Vitae Germany
Historisches Institut Dr. Alexander Schmidt Akademischer Rat Universität Jena · Einrichtung · 07737 Jena Fürstengraben 13 07743 Jena Curriculum Vitae Germany Telefon: +49 36 41 9-4979 Telefax: +49 36 41 9-310 02 E-Mail: [email protected] ACADEMIC DEGREES Jena, 29. Februar 2020 M.A. (1,0, 2001, Friedrich Schiller University of Jena), Dr.phil. (summa cum laude, 2005, Friedrich Schiller University of Jena) ACADEMIC POSITION Akademischer Rat, Historisches Institut, Friedrich Schiller University of Jena PREVIOUS POSITIONS 07/2009-03/2017 Juniorprofessor of Intellectual History, Research Centre “Laboratory Enlightenment”, Friedrich Schiller University of Jena 10/2004-12/2008 Research fellow at the Collaborative Research Centre 482 “The Weimar-Jena phenomenon: Culture around 1800” (Sonderforschungsbereich 482 “Ereignis Weimar-Jena. Kultur um 1800”), Friedrich Schiller University of Jena 7/2001–1/2002 Research fellow at the Department of History (Early Modern Studies), Friedrich Schiller University of Jena GRANTS, AWARDS, SCHOLARSHIPS 7/2018 (together with Professor Paul Cheney, University of Chicago) Summer Institute for University and College Teachers sponsored by the National Endowment for the Humanities ($ 110,694), Visiting Faculty at University of Chicago 12/2017 Initial grant by the Centre for the Study of the Global Condition (Leipzig) for the “Human rights without religion?” project (5500 €) 7/2015-11/2016 Feodor-Lynen-Fellowship for Experienced Researchers (offered by the Alexander von Humboldt-Foundation) at the John U. -

The Berlin Group and the Philosophy of Logical Empiricism BOSTON STUDIES in the PHILOSOPHY and HISTORY of SCIENCE
The Berlin Group and the Philosophy of Logical Empiricism BOSTON STUDIES IN THE PHILOSOPHY AND HISTORY OF SCIENCE Editors ROBERT S. COHEN, Boston University JURGEN¨ RENN, Max Planck Institute for the History of Science KOSTAS GAVROGLU, University of Athens Managing Editor LINDY DIVARCI, Max Planck Institute for the History of Science Editorial Board THEODORE ARABATZIS, University of Athens ALISA BOKULICH, Boston University HEATHER E. DOUGLAS, University of Pittsburgh JEAN GAYON, Universit´eParis1 THOMAS F. GLICK, Boston University HUBERT GOENNER, University of Goettingen JOHN HEILBRON, University of California, Berkeley DIANA KORMOS-BUCHWALD, California Institute of Technology CHRISTOPH LEHNER, Max Planck Institute for the History of Science PETER MCLAUGHLIN, Universit¨at Heidelberg AGUSTI´ NIETO-GALAN, Universitat Aut`onoma de Barcelona NUCCIO ORDINE, Universit´a della Calabria ANA SIMOES,˜ Universidade de Lisboa JOHN J. STACHEL, Boston University SYLVAN S. SCHWEBER, Harvard University BAICHUN ZHANG, Chinese Academy of Science VOLUME 273 For further volumes: http://www.springer.com/series/5710 Nikolay Milkov • Volker Peckhaus Editors The Berlin Group and the Philosophy of Logical Empiricism 123 Editors Nikolay Milkov Volker Peckhaus Department of Philosophy Department of Philosophy University of Paderborn University of Paderborn 33098 Paderborn 33098 Paderborn Germany Germany ISSN 0068-0346 ISBN 978-94-007-5484-3 ISBN 978-94-007-5485-0 (eBook) DOI 10.1007/978-94-007-5485-0 Springer Dordrecht Heidelberg New York London Library of Congress -
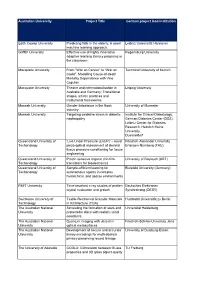
Australian University Project Title German Project Lead Institution
Australian University Project Title German project lead institution Edith Cowan University Predicting falls in the elderly: A novel Leibniz Universität Hannover machine learning approach. Griffith University Effective use of highly innovative Regensburg University adaptive learning literacy programs in the classroom Macquarie University From “War on Cancer” to “War on Technical University of Munich Covid”: Modelling Cause-of-death Mortality Dependence with Vine Copulas Macquarie University Theatre and internationalisation in Leipzig University Australia and Germany: Transitional stages, artistic practices and institutional frameworks Monash University Gender Imbalance in the Book University of Muenster Industry Monash University Targeting oxidative stress in diabetic Institute for Clinical Diabetology, nephropathy German Diabetes Center (DDZ), Leibniz Center for Diabetes Research, Heinrich Heine University, Duesseldorf Queensland University of ‘Live Under Pressure (LivUP)’ – novel Friedrich-Alexander University Techonology piezo-optical assessment of skeletal Erlangen-Nürnberg (FAU) tissue pressure-conditioning for tissue engineering Queensland University of Proton sensitive organic thin film University of Bayreuth (UBT) Techonology transistors for bioelectronics Queensland University of Sample-efficient learning for Bielefeld University (Germany) Techonology autonomous agents in complex hierarchical, and sparse environments RMIT University Time-resolved x-ray studies of protien Deutsches Elektronen crystal nucleation and growth Synchrotrong -

Frege in Context
British Journal for the History of Philosophy 9(3) 2001:557– 570 REVIEW ARTICLE FREGE IN CONTEXT Nikolay Milkov Gottfried Gabriel und Wolfgang Kienzler (eds). Frege in Jena. Beiträge zur Spurensicherung . (Kritisches Jahrbuch der Philosophie , Band 2). Würzburg, Königshausen & Neumann, 1997, pp.161. Pb. DM 28. ISBN 3826014405 Gottfried Gabriel und Uwe Dathe (eds). Gottlob Frege. Werk und Wirkung. Paderborn, Mentis, 2000, pp.313. Pb. DM 30. ISBN 3897850850 REWRITING FREGE’S BIOGRAPHY English-speaking philosophers know little about Frege’s life. In 1972 T. W. Bynum published ‘On the Life and Work of Gottlob Frege’, an introduction of fty-four pages to his translation of the Begriffsschrift , in which various facts about Frege’s life were cited. These notes are still being used as a guide to Frege’s biography today – as in Anthony Kenny’s recent book Frege (see Kenny 1995, p. 1 n.). Apparently, many writers on Frege have never heard about a complete revolution in investigating Frege’s biography that took place in the 1990s in Germany, falsifying almost every sentence in Bynum’s piece. The two volumes under review – I shall refer to them as Spurensicherung and Wirkung for brevity – which are the outcome of two symposia on Frege held at the University of Jena, form the pinnacle of this biographical makeover. Indeed, from 1979 on, four such symposia had already taken place in Jena, resulting in three volumes of proceedings in each of which new data pertaining to Frege’s biography were contained. What is new in 1 I use the term ‘East German’ here, following the tradition established during the Cold War, when Germany was divided into two parts, usually known in English as East and West Germany. -

International Conference on Cross Movement Mobilization 05 – 08 April 2017, Bochum
International Conference on Cross Movement Mobilization 05 – 08 April 2017, Bochum Wednesday, April 5, 2017 Location: ISB 16:10 Entrance 16:40-17:30 Welcome Address Stefan Berger (ISB/ RUB), Sabrina Zajak (ISB/RUB), Geoffrey Pleyers (ISA 47), Simon Teune (IPB), Jens Becker(HBS) 17:30-19:30 Opening Discussion Cross-Movement Mobilization as a Conceptual and Practical Challenge Donatella della Porta, Centre of Social Movements Studies; Scuola Normale Superior Florence Dieter Rucht, Protest and social Movement Research Institute; Berlin Social Science Center Peter Evans, University of California, Berkeley; Watson Institute for International Studies and Public Affairs, Brown University 19:30- 20:00 Reception 20:00- open end Drink and think: Poster Session Thursday, April 6, 2017 Location: Ruhr-University Bochum / HIC 08:30-10:00 Morning plenary Theorizing cross movement alliances: social movement, labour and postcolonial studies in dialogue Klaus Dörre, University of Jena, Germany Mario Diani, University of Trento, Italy Janet M. Conway, Canada research Chair in Social Justice, Brock University, Canada Chair: Geoffrey Pleyers, Université catholique de Louvain, Belgium Panel Slot A Room: Panel 9.1. Cross-Movement Convergences: The Urban as Opportunity or Limitation? IC 03/604 Organizers: Margit Mayer, David Scheller Chair/Discussant: Margit Mayer 1. Justus Uitermark (University of Amsterdam): The urban vortex. Connections across cities and movements 2. Nina Fraeser (Hafen-City Uni Hamburg): Commoning as solidarity practice: the social-spatial reproduction of urban social movements 3. David Scheller (FH Potsdam): Beyond housing movements? Convergences of urban social movements in Berlin and New York Room: Panel 7.1: The Next Polanyian Movement? Mobilization in Times of Global Capitalism. -

Projects SPACES II Cabude
Projects SPACES II CaBuDe Acronym Title Section Coordinator Email Partners in Germany Partners in Africa - INIP Luanda/Angola; - University of Hamburg Benguela Niños: Physical processes and - NatMIRC Swakopmund/Namibia; BANINO marine Prof. Peter Brandt, GEOMAR [email protected] - Leibniz Institute for Baltic Sea Research, Warnemünde (IOW) long-term variability - Gobabeb Research and Training Centre/Namibia - Helmholtz Centre for Ocean Research, Kiel (GEOMAR) - University of Cape Town/South Africa - University of Cape Town/South Africa - University of Siegen - Council for Scientific and Industrial Research (CSIR)/South Changes in the Agulhas System and its CASISAC marine Prof. Arne Biastoch, GEOMAR [email protected] - University of Kiel Africa Impact on Southern African Coasts - Helmholtz Centre for Ocean Research, Kiel (GEOMAR) - South African Environmental Observation Network (SAEON)/South Africa - University of KwaZulu-Natal, Durban/South Africa - University of Jena Tracing Human and Climate Impacts in Dr. Matthias Zabel, University of - University of the Witwatersrand, Johannesburg/South Africa TRACES marine [email protected] - University of Greifswald South Africa Bremen - Nelson Mandela University, Port Elizabeth/South Africa - University of Bremen - Council for Geoscience, Cape Town/South Africa - University of Bremen - University of Cape Town/South Africa Trophic transfer efficiency in Benguela - University of Hamburg - DAFF Cape Town/South Africa TRAFFIC marine Dr. Werner Ekau, ZMT [email protected] current - Thünen -

LANTERN 2021 Proceedings of the Third Workshop on Beyond Vision
LANTERN 2021 Proceedings of the Third Workshop on Beyond Vision and LANguage: inTEgrating Real-world kNowledge (LANTERN) April 20, 2021 Kyiv, Ukraine (Online) ©2021 The Association for Computational Linguistics Order copies of this and other ACL proceedings from: Association for Computational Linguistics (ACL) 209 N. Eighth Street Stroudsburg, PA 18360 USA Tel: +1-570-476-8006 Fax: +1-570-476-0860 [email protected] ISBN 978-1-954085-15-2 ii Introduction Welcome to LANTERN 2021, the Third Workshop Beyond Vision and Language: Integrating Real- World Knowledge, co-located with EACL 2021. Building on the successes of the first edition co-located with EMNLP-IJCNLP 2019 and the second edition co-located with COLING 2020, the third edition of LANTERN aims to again bring together and interconnect researchers focusing on natural language research from a multimodal perspective. In particular, the main goal of the workshop is to promote and foster research which uses machine- and deep-learning techniques to interconnect language with vision and other modalities by leveraging external knowledge. We encourage contributions which exploit very diverse sources of external knowledge like knowledge graphs, fixed and dynamic environments, cognitive and neuroscience data, etc. This results in the workshop being open to all research directions which acknowledge the importance of knowledge in acquiring, using, and evaluating language in real-world settings. In this third edition, we called for both long and short papers. All the accepted contributions are published in these Proceedings. LANTERN 2021 received 11 submissions, all of which were double-blindly reviewed by three highly-qualified reviewers. In total, 5 papers (4 long, 1 short) were accepted to appear in the Proceedings of the workshop, with an acceptance rate of around 55% (comparable to that of the first and second edition, which was around 53% and 57%, respectively). -

University of Paderborn Warburger Strasse 100 33098 Paderborn / Germany Causal Linkages Between Domestic Terrorism and Economic Growth
Working Paper Series No. 2009-02 Causal Linkages Between Domestic Terrorism and Economic Growth Thomas Gries, Tim Krieger and Daniel Meierrieks February 2009 Center for International Economics University of Paderborn Warburger Strasse 100 33098 Paderborn / Germany Causal Linkages Between Domestic Terrorism and Economic Growth Thomas Gries, Tim Kriegery, Daniel Meierrieksz February 17, 2009 Abstract We use the Hsiao-Granger method to test for growth-terrorism causality for seven Western European countries. In bivariate settings, the impact of economic performance on domestic terrorism is very strong. In trivariate settings, the impact of performance on terrorism diminishes. Here, we …nd that economic performance leads terrorist violence in robust ways only for three out of seven countries. Terrorism is almost never found to causally in‡uence growth in bivariate and trivariate speci…cations. Our …ndings indicate that (i) the role of economic performance in determining terrorist violence appears to have been important for some countries and (ii) all attacked economies have been successful in adjusting to the threat of terrorism. Keywords: Domestic Terrorism, Growth, Hsiao-Granger Causality, West- ern Europe JEL Classi…cation: C32, N40, 052 Corresponding Author. University of Paderborn, Department of Economics, Warburger Straße100, 33098 Paderborn. Ph.: +49-(0)5251-60-2113, Fax: +49-(0)5251-60-3540, E-mail: [email protected]. yUniversity of Paderborn, Department of Economics, Warburger Straße 100, 33098 Paderborn, Germany. Ph.: +49-(0)5251-60-2117, Fax: +49-(0)5251-60-5005, E-mail: [email protected]. zUniversity of Paderborn, Department of Economics, Warburger Straße 100, 33098 Paderborn, Germany, Ph.: +49-(0)5251-60-2115, Fax: +49-(0)5251-60-2115, E-mail: [email protected].