Gene Expression and Regulation in Early Vertebrate Development Xianhui Li
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differences in Molecular Regulation Between Osteochondroma and Bizarre Parosteal Osteochondromatous Proliferation
MOLECULAR MEDICINE REPORTS 16: 801-805, 2017 Differences in molecular regulation between osteochondroma and bizarre parosteal osteochondromatous proliferation XINRONG ZHOU, LIHUI DENG, XINSHENG HAN, YI CHEN, JIAO WANG and SHENGNAN DU Department of Stomatology, Nanchong Central Hospital, Nanchong, Sichuan 637000, P.R. China Received April 13, 2016; Accepted March 24, 2017 DOI: 10.3892/mmr.2017.6634 Abstract. The differences in molecular mechanisms between exhibit a cauliflower-like shape. Histologically, there is a osteochondroma and bizarre parosteal osteochondromatous fibrous perichondrium, which covers the cartilage cap and proliferation (BPOP) remain to be fully elucidated. In the exhibits continuity with the periosteum of the underlying bone present study, the differentially expressed genes between marrow. Bizarre parosteal osteochondromatous proliferation BPOP and osteochondroma were obtained from the Gene (BPOP) is a rare, benign osteocartilaginous lesion, which Expression Omnibus online database, and the associations can occur in the hands, feet, zygoma, maxilla and mandible. among these genes were analyzed using the Database for The histological features of BPOP include osteocartilaginous Annotation, Visualization, and Integrated Discovery (DAVID) interfaces, a scattering of bizarre enlarged chondrocytes and online bioinformatics software. The results revealed several hypercellular spindle cells (1,3). Previous studies have shown differentially expressed genes between human BPOP and that BPOP arises from periosteal tissues through -

Latent TGF-Beta Binding Protein-1 Plays an Important Role in Craniofacial Development
Original Article http://dx.doi.org/10.1590/1678-7757-2020-0262 Latent TGF-beta binding protein-1 plays an important role in craniofacial development Abstract Yiting XIONG1# Objective: This study aims to replicate the phenotype of Ltbp1 knockout mice in zebrafish, and to address the function of LTBP1 in craniofacial Rongrong SUN1# development. Methods: Whole mount in situ hybridization (WISH) of ltbp1 Jingyu LI2 was performed at critical periods of zebrafish craniofacial development to Yue WU2 explore the spatial-temporal expression pattern. Furthermore, we generated 1 Jingju ZHANG morpholino based knockdown model of ltbp1 to study the craniofacial phenotype. Results: WISH of ltbp1 was mainly detected in the mandibular jaw region, brain trunk, and internal organs such as pancreas and gallbladder. And ltbp1 colocalized with both sox9a and ckma in mandibular region. Morpholino based knockdown of ltbp1 results in severe jaw malformation. Alcian blue staining revealed severe deformity of Meckel’s cartilage along with the absence of ceratobranchial. Three-dimension measurements of ltbp1 morphants jaws showed decrease in both mandible length and width and increase in open mouth distance. Expression of cartilage marker sox9a and muscle marker ckma was decreased in ltbp1 morphants. Conclusions: Our experiments found that ltbp1 was expressed in zebrafish mandibular jaw cartilages and the surrounding muscles. The ltbp1 knockdown zebrafish exhibited phenotypes consistent with Ltbp1 knockout mice. And loss of ltbp1 function lead to significant mandibular jaw defects and affect both jaw cartilages and surrounding muscles. Keywords: LTBP1. Craniofacial anomalies. Developmental biology. Zebrafish. Submitted: April 24, 2020 Modification: June 31, 2020 Accepted: July 29, 2020 Corresponding address: ¹Tongji University, Shanghai Engineering Research Center of Tooth Restoration and Regeneration, Jingju Zhang Department of Orthodontics, School & Hospital of Stomatology, Shanghai, China. -

LTBP4 in Health and Disease
G C A T T A C G G C A T genes Review LTBP4 in Health and Disease Chi-Ting Su 1,2,3 and Zsolt Urban 2,* 1 Department of Internal Medicine, Renal Division, National Taiwan University Hospital Yunlin Branch, Douliu 640, Taiwan; [email protected] 2 Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA 15261, USA 3 Department of Medicine, National Taiwan University Cancer Center Hospital, Taipei 106, Taiwan * Correspondence: [email protected]; Tel.: +1-412-648-8269 Abstract: Latent transforming growth factor β (TGFβ)-binding protein (LTBP) 4, a member of the LTBP family, shows structural homology with fibrillins. Both these protein types are characterized by calcium-binding epidermal growth factor-like repeats interspersed with 8-cysteine domains. Based on its domain composition and distribution, LTBP4 is thought to adopt an extended structure, facilitating the linear deposition of tropoelastin onto microfibrils. In humans, mutations in LTBP4 result in autosomal recessive cutis laxa type 1C, characterized by redundant skin, pulmonary emphysema, and valvular heart disease. LTBP4 is an essential regulator of TGFβ signaling and is related to development, immunity, injury repair, and diseases, playing a central role in regulating inflammation, fibrosis, and cancer progression. In this review, we focus on medical disorders or diseases that may be manipulated by LTBP4 in order to enhance the understanding of this protein. Keywords: LTBP4; TGFβ; elastogenesis; medicine 1. Introduction Citation: Su, C.-T.; Urban, Z. LTBP4 Latent transforming growth factor β-binding proteins (LTBPs) serve as mediators to in Health and Disease. Genes 2021, 12, organize elements for matrix microfibril bundles and regulate the signaling of transforming 795. -

Instruction Manual for RESEARCH USE ONLY NOT for USE in CLINICAL DIAGNOSTIC PROCEDURES
APA124Ra01 50µg Active Transforming Growth Factor Beta 1 (TGFb1) Organism Species: Rattus norvegicus (Rat) Instruction manual FOR RESEARCH USE ONLY NOT FOR USE IN CLINICAL DIAGNOSTIC PROCEDURES 1st Edition (Apr, 2016) [ PROPERTIES ] Source: Prokaryotic expression. Host: E. coli Residues: Ala279~Ser390 Tags: N-terminal His-tag Purity: >95% Buffer Formulation: 20mM Tris, 150mM NaCl, pH8.0, containing 0.05% sarcosyl and 5% trehalose. Applications: Cell culture; Activity Assays. (May be suitable for use in other assays to be determined by the end user.) Predicted isoelectric point: 8.4 Predicted Molecular Mass: 14.1kDa Accurate Molecular Mass: 16kDa as determined by SDS-PAGE reducing conditions. [ USAGE ] Reconstitute in 20mM Tris, 150mM NaCl (pH8.0) to a concentration of 0.1-1.0 mg/mL. Do not vortex. [ STORAGE AND STABILITY ] Storage: Avoid repeated freeze/thaw cycles. Store at 2-8oC for one month. Aliquot and store at -80oC for 12 months. Stability Test: The thermal stability is described by the loss rate. The loss rate was determined by accelerated thermal degradation test, that is, incubate the protein at 37oC for 48h, and no obvious degradation and precipitation were observed. The loss rate is less than 5% within the expiration date under appropriate storage condition. [ SEQUENCE ] [ ACTIVITY ] Transforming Growth Factor Beta 1 (TGFb1) is a multifunctional set of peptides that controls proliferation, differentiation, and other functions in many cell types. TGF beta 1 has been shown to interact with TGF beta receptor 1, Decorin, LTBP1 and so on. Besides, Latent Transforming Growth Factor Beta Binding Protein 1 (LTBP1) has been identified as an interactor of TGFb1, thus a binding ELISA assay was conducted to detect the interaction of recombinant rat TGFb1 and recombinant mouse LTBP1 (high homology with rat). -

Modulation of Skeletal Muscle Insulin Signaling with Chronic Caloric Restriction in Cynomolgus Monkeys
Diabetes Publish Ahead of Print, published online March 31, 2009 Modulation of skeletal muscle insulin signaling with chronic caloric restriction in cynomolgus monkeys Zhong Q. Wang1 Elizabeth Floyd1 Jianhua Qin1 Xiaotuan Liu1 Yongmei Yu1 Xian H Zhang1 Janice D. Wagner2 William T. Cefalu1 Division of Nutrition and Chronic Diseases1, Pennington Biomedical Research Center, Louisiana State University System; Department of Pathology, Wake Forest University School of Medicine 2 Corresponding Author: William T Cefalu, M.D. Email: [email protected] Submitted 18 July 2008 and accepted 19 March 2009. This is an uncopyedited electronic version of an article accepted for publication in Diabetes. The American Diabetes Association, publisher of Diabetes, is not responsible for any errors or omissions in this version of the manuscript or any version derived from it by third parties. The definitive publisher-authenticated version will be available in a future issue of Diabetes in print and online at http://diabetes.diabetesjournals.org. 1 Copyright American Diabetes Association, Inc., 2009 Objective: Caloric restriction (CR) has been shown to retard aging processes, extend maximal life span, and consistently increase insulin action in experimental animals. The mechanism by which CR enhances insulin action, specifically in higher species, is not precisely known. We sought to examine insulin receptor signaling and transcriptional alterations in skeletal muscle of nonhuman primates subjected to caloric restriction over a 4 year period. Research Design: After baseline, 32 male adult cynomolgus monkeys (Macaca fascicularis) were randomized to an Ad libitum diet (AL) or to 30% CR. Dietary intake, body weight and insulin sensitivity were obtained at routine intervals over 4 years. -

A Novel Gene Expression Profile in Lymphatics Associated with Tumor
Research Article A Novel Gene Expression Profile in Lymphatics Associated with Tumor Growth and Nodal Metastasis Steven Clasper,1 Daniel Royston,1 Dilair Baban,2 Yihai Cao,3 Stephan Ewers,4 Stefan Butz,4 Dietmar Vestweber,4 and David G. Jackson1 1MRC Human Immunology Unit, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital; 2MRC Functional Genetics Unit, Department of Human Anatomy and Genetics, Oxford, United Kingdom; 3Microbiology and Tumor Biology Centre, Karolinska Institute, Stockholm, Sweden; and 4Max Planck Institute for Molecular Biomedicine, Mu¨nster, Germany Abstract would be desirable either as independent therapies or as adjuncts Invasion of lymphatic vessels is a key step in the metastasis of to existing chemotherapy (1). However, the ability to develop such primary tumors to draining lymph nodes. Although the approaches is currently handicapped by poor understanding of process is enhanced by tumor lymphangiogenesis, it is unclear lymphatic vessel molecular biology and ignorance of the molecular whether this is a consequence of increased lymphatic vessel interactions that occur between migrating cells and lymphatic number, altered lymphatic vessel properties, or both. Here we vessel endothelium (see ref. 2 for recent review). This contrasts have addressed the question by comparing the RNA profiles of with the blood vascular system, where at least the basic primary lymphatic endothelial cells (LEC) isolated from the mechanisms regulating vessel wall transmigration have been well vasculature of normal tissue and from highly metastatic studied and extensively characterized (3). The target vessels for invasion by lymph-metastasizing tumor T-241/vascular endothelial growth factor (VEGF)-C fibrosar- comas implanted in C57BL/6 mice. -

Inhibiting Bone Morphogenetic Protein 4 Type I Receptor Signaling Promotes Remyelination by Potentiating Oligodendrocyte Differentiation
New Research Disorders of the Nervous System Inhibiting Bone Morphogenetic Protein 4 Type I Receptor Signaling Promotes Remyelination by Potentiating Oligodendrocyte Differentiation Alistair E. Govier-Cole, Rhiannon J. Wood, Jessica L. Fletcher, David G. Gonsalvez, Daniel Merlo, Holly S. Cate, Simon S. Murray, and Junhua Xiao https://doi.org/10.1523/ENEURO.0399-18.2019 Department of Anatomy and Neuroscience, University of Melbourne, Parkville 3010, Victoria, Australia Visual Abstract Significance Statement Blocking inhibitory factors within central demyelinating lesions is a promising strategy to promote remyeli- nation. Previous studies have established that exogenous bone morphogenetic protein (BMPs) inhibit oligodendrocyte differentiation during CNS development and after injury. Here, we demonstrate that blocking endogenous BMP4 signaling via a selective pharmacological approach promotes oligodendroglial differentiation and the rate of remyelination after a central demyelinating insult in vivo. Using in vitro analysis, we identify that oligodendrocyte progenitor cell (OPC)-expressed BMP Type I receptors mediate this effect. Together, our data propose that blocking the BMP4 signaling pathway at the Type I receptors in OPCs is a promising strategy to promote CNS remyelination. March/April 2019, 6(2) e0399-18.2019 1–22 New Research 2 of 22 Blocking inhibitory factors within CNS demyelinating lesions is regarded as a promising strategy to promote remyelination. Bone morphogenetic protein 4 (BMP4) is an inhibitory factor present in demyelinating lesions. Noggin, an endogenous antagonist to BMP, has previously been shown to increase the number of oligodendro- cytes and promote remyelination in vivo. However, it remains unclear how BMP4 signaling inhibits remyelination. Here we investigated the downstream signaling pathway that mediates the inhibitory effect that BMP4 exerts upon remyelination through pharmacological and transgenic approaches. -

Mirna Replacement in Aortic Calcification Ying Tang1, †, Tapan A
MicroRNA profiles in calcified and healthy aorta: therapeutic impact of miR-145 and miR-378 Running title: miRNA replacement in aortic calcification Ying Tang1, †, Tapan A. Shah1, †, ‡, Edward J. Yurkow2, and Melissa B. Rogers1, ‡ 1Rutgers - New Jersey Medical School, Microbiology, Biochemistry, & Molecular Genetics, Newark, NJ 2Rutgers University Molecular Imaging Center (RUMIC), Rutgers University, Piscataway, NJ †Co-first authors ‡Present Address: Advanced Cell Diagnostics, 7707 Gateway Blvd #200, Newark, CA 94560 §To whom should correspondence and reprint request be addressed to: Melissa B. Rogers, Ph.D., Microbiology, Biochemistry & Molecular Genetics, Rutgers - NJ Medical School (NJMS), Center for Cell Signaling, Room F1216, 205 South Orange Ave., Newark, NJ 07103. Email: [email protected], telephone: 973 972 2984 Fig. S1. Experimental Controls. Fig. S2. KEGG (Kyoto Encyclopedia of Genes and Genomes) analyses of differentially expressed miRNAs in male Klotho homozygous mutant mice. Fig. S3. KEGG (Kyoto Encyclopedia of Genes and Genomes) analyses of differentially expressed miRNAs in female Klotho homozygous mutant mice. Fig. S4. Gene Ontology (GO) analyses of differentially expressed miRNAs in male Klotho homozygous mutant mice. Fig. S5. Gene Ontology (GO) analyses of differentially expressed miRNAs in female Klotho homozygous mutant mice. Fig. S6. Interaction network of miRNAs down-regulated in male Klotho homozygotes relative to healthy controls. Fig. S7. Interaction network of miRNAs down-regulated in female Klotho homozygotes relative to healthy controls. Table S1. Klotho wild type and Klotho heterozygous mice are equivalent controls. Table S2. Average miRNA abundance in aorta from Klotho mutant homozygotes relative to healthy control (p<0.05). Table S3. Genes targeted by selected miRNAs that may influence aortic calcification. -

Mining the Plasma-Proteome Associated Genes in Patients with Gastro-Esophageal Cancers for Biomarker Discovery
www.nature.com/scientificreports OPEN Mining the plasma‑proteome associated genes in patients with gastro‑esophageal cancers for biomarker discovery Frederick S. Vizeacoumar1, Hongyu Guo2, Lynn Dwernychuk3, Adnan Zaidi3,4, Andrew Freywald1, Fang‑Xiang Wu2,5,6, Franco J. Vizeacoumar3,4,8* & Shahid Ahmed3,4,7* Gastro‑esophageal (GE) cancers are one of the major causes of cancer‑related death in the world. There is a need for novel biomarkers in the management of GE cancers, to yield predictive response to the available therapies. Our study aims to identify leading genes that are diferentially regulated in patients with these cancers. We explored the expression data for those genes whose protein products can be detected in the plasma using the Cancer Genome Atlas to identify leading genes that are diferentially regulated in patients with GE cancers. Our work predicted several candidates as potential biomarkers for distinct stages of GE cancers, including previously identifed CST1, INHBA, STMN1, whose expression correlated with cancer recurrence, or resistance to adjuvant therapies or surgery. To defne the predictive accuracy of these genes as possible biomarkers, we constructed a co‑expression network and performed complex network analysis to measure the importance of the genes in terms of a ratio of closeness centrality (RCC). Furthermore, to measure the signifcance of these diferentially regulated genes, we constructed an SVM classifer using machine learning approach and verifed these genes by using receiver operator characteristic (ROC) curve as an evaluation metric. The area under the curve measure was > 0.9 for both the overexpressed and downregulated genes suggesting the potential use and reliability of these candidates as biomarkers. -

Stromal Modulators of TGF- in Cancer
Journal of Clinical Medicine Review Stromal Modulators of TGF-β in Cancer Brunella Costanza 1,†, Ijeoma Adaku Umelo 1,†, Justine Bellier 1,†, Vincent Castronovo 1 and Andrei Turtoi 1,2,* 1 Metastasis Research Laboratory, GIGA-Cancer, University of Liege, 4000 Liege, Belgium; [email protected] (B.C.); [email protected] (I.A.U.); [email protected] (J.B.); [email protected] (V.C.) 2 Institut de Recherche en Cancérologie de Montpellier (IRCM), INSERM U1194, Université Montpellier, Institut Régional du Cancer de Montpellier, 34298 Montpellier, France * Correspondence: [email protected]; Tel.: +33-467-61-3746 † These authors contributed equally to this work. Academic Editor: Emanuel F. Petricoin Received: 4 November 2016; Accepted: 23 December 2016; Published: 6 January 2017 Abstract: Transforming growth factor-β (TGF-β) is an intriguing cytokine exhibiting dual activities in malignant disease. It is an important mediator of cancer invasion, metastasis and angiogenesis, on the one hand, while it exhibits anti-tumor functions on the other hand. Elucidating the precise role of TGF-β in malignant development and progression requires a better understanding of the molecular mechanisms involved in its tumor suppressor to tumor promoter switch. One important aspect of TGF-β function is its interaction with proteins within the tumor microenvironment. Several stromal proteins have the natural ability to interact and modulate TGF-β function. Understanding the complex interplay between the TGF-β signaling network and these stromal proteins may provide greater insight into the development of novel therapeutic strategies that target the TGF-β axis. The present review highlights our present understanding of how stroma modulates TGF-β activity in human cancers. -

SUPPORTING INFORMATION for Regulation of Gene Expression By
SUPPORTING INFORMATION for Regulation of gene expression by the BLM helicase correlates with the presence of G4 motifs Giang Huong Nguyen1,2, Weiliang Tang3, Ana I. Robles1, Richard P. Beyer4, Lucas T. Gray5, Judith A. Welsh1, Aaron J. Schetter1, Kensuke Kumamoto1,6, Xin Wei Wang1, Ian D. Hickson2,7, Nancy Maizels5, 3,8 1 Raymond J. Monnat, Jr. and Curtis C. Harris 1Laboratory of Human Carcinogenesis, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, U.S.A; 2Department of Medical Oncology, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, University of Oxford, Oxford, U.K.; 3Department of Pathology, University of Washington, Seattle, WA U.S.A.; 4 Center for Ecogenetics and Environmental Health, University of Washington, Seattle, WA U.S.A.; 5Department of Immunology and Department of Biochemistry, University of Washington, Seattle, WA U.S.A.; 6Department of Organ Regulatory Surgery, Fukushima Medical University, Fukushima, Japan; 7Cellular and Molecular Medicine, Nordea Center for Healthy Aging, University of Copenhagen, Denmark; 8Department of Genome Sciences, University of WA, Seattle, WA U.S.A. SI Index: Supporting Information for this manuscript includes the following 19 items. A more detailed Materials and Methods section is followed by 18 Tables and Figures in order of their appearance in the manuscript text: 1) SI Materials and Methods 2) Figure S1. Study design and experimental workflow. 3) Figure S2. Immunoblot verification of BLM depletion from human fibroblasts. 4) Figure S3. PCA of mRNA and miRNA expression in BLM-depleted human fibroblasts. 5) Figure S4. qPCR confirmation of mRNA array data. 6) Table S1. BS patient and control detail. -
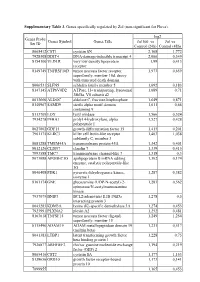
Supplementary Table 3. Genes Specifically Regulated by Zol (Non-Significant for Fluva)
Supplementary Table 3. Genes specifically regulated by Zol (non-significant for Fluva). log2 Genes Probe Genes Symbol Genes Title Zol100 vs Zol vs Set ID Control (24h) Control (48h) 8065412 CST1 cystatin SN 2,168 1,772 7928308 DDIT4 DNA-damage-inducible transcript 4 2,066 0,349 8154100 VLDLR very low density lipoprotein 1,99 0,413 receptor 8149749 TNFRSF10D tumor necrosis factor receptor 1,973 0,659 superfamily, member 10d, decoy with truncated death domain 8006531 SLFN5 schlafen family member 5 1,692 0,183 8147145 ATP6V0D2 ATPase, H+ transporting, lysosomal 1,689 0,71 38kDa, V0 subunit d2 8013660 ALDOC aldolase C, fructose-bisphosphate 1,649 0,871 8140967 SAMD9 sterile alpha motif domain 1,611 0,66 containing 9 8113709 LOX lysyl oxidase 1,566 0,524 7934278 P4HA1 prolyl 4-hydroxylase, alpha 1,527 0,428 polypeptide I 8027002 GDF15 growth differentiation factor 15 1,415 0,201 7961175 KLRC3 killer cell lectin-like receptor 1,403 1,038 subfamily C, member 3 8081288 TMEM45A transmembrane protein 45A 1,342 0,401 8012126 CLDN7 claudin 7 1,339 0,415 7993588 TMC7 transmembrane channel-like 7 1,318 0,3 8073088 APOBEC3G apolipoprotein B mRNA editing 1,302 0,174 enzyme, catalytic polypeptide-like 3G 8046408 PDK1 pyruvate dehydrogenase kinase, 1,287 0,382 isozyme 1 8161174 GNE glucosamine (UDP-N-acetyl)-2- 1,283 0,562 epimerase/N-acetylmannosamine kinase 7937079 BNIP3 BCL2/adenovirus E1B 19kDa 1,278 0,5 interacting protein 3 8043283 KDM3A lysine (K)-specific demethylase 3A 1,274 0,453 7923991 PLXNA2 plexin A2 1,252 0,481 8163618 TNFSF15 tumor necrosis