Factors Controlling Hypoxia Occurrence in Estuaries, Chester River, Chesapeake Bay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2012-AG-Environmental-Audit.Pdf
TABLE OF CONTENTS INTRODUCTION .............................................................................................................. 1 CHAPTER ONE: YOUGHIOGHENY RIVER AND DEEP CREEK LAKE .................. 4 I. Background .......................................................................................................... 4 II. Active Enforcement and Pending Matters ........................................................... 9 III. The Youghiogheny River/Deep Creek Lake Audit, May 16, 2012: What the Attorney General Learned............................................................................................. 12 CHAPTER TWO: COASTAL BAYS ............................................................................. 15 I. Background ........................................................................................................ 15 II. Active Enforcement Efforts and Pending Matters ............................................. 17 III. The Coastal Bays Audit, July 12, 2012: What the Attorney General Learned .. 20 CHAPTER THREE: WYE RIVER ................................................................................. 24 I. Background ........................................................................................................ 24 II. Active Enforcement and Pending Matters ......................................................... 26 III. The Wye River Audit, October 10, 2012: What the Attorney General Learned 27 CHAPTER FOUR: POTOMAC RIVER NORTH BRANCH AND SAVAGE RIVER 31 I. Background ....................................................................................................... -

Maryland Stream Waders 10 Year Report
MARYLAND STREAM WADERS TEN YEAR (2000-2009) REPORT October 2012 Maryland Stream Waders Ten Year (2000-2009) Report Prepared for: Maryland Department of Natural Resources Monitoring and Non-tidal Assessment Division 580 Taylor Avenue; C-2 Annapolis, Maryland 21401 1-877-620-8DNR (x8623) [email protected] Prepared by: Daniel Boward1 Sara Weglein1 Erik W. Leppo2 1 Maryland Department of Natural Resources Monitoring and Non-tidal Assessment Division 580 Taylor Avenue; C-2 Annapolis, Maryland 21401 2 Tetra Tech, Inc. Center for Ecological Studies 400 Red Brook Boulevard, Suite 200 Owings Mills, Maryland 21117 October 2012 This page intentionally blank. Foreword This document reports on the firstt en years (2000-2009) of sampling and results for the Maryland Stream Waders (MSW) statewide volunteer stream monitoring program managed by the Maryland Department of Natural Resources’ (DNR) Monitoring and Non-tidal Assessment Division (MANTA). Stream Waders data are intended to supplementt hose collected for the Maryland Biological Stream Survey (MBSS) by DNR and University of Maryland biologists. This report provides an overview oft he Program and summarizes results from the firstt en years of sampling. Acknowledgments We wish to acknowledge, first and foremost, the dedicated volunteers who collected data for this report (Appendix A): Thanks also to the following individuals for helping to make the Program a success. • The DNR Benthic Macroinvertebrate Lab staffof Neal Dziepak, Ellen Friedman, and Kerry Tebbs, for their countless hours in -

Organic Compounds and Trace Elements in the Pocomoke River and Tributaries, Maryland
Organic Compounds and Trace Elements in the Pocomoke River and Tributaries, Maryland Open-File Report 99-57 (Revised January 2000) In cooperation with George Mason University and Maryland Department of the Environment U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY Organic Compounds and Trace Elements in the Pocomoke River and Tributaries, Maryland By Cherie V. Miller, Gregory D. Foster, Thomas B. Huff, and John R. Garbarino Open-File Report 99-57 (Revised January 2000) In cooperation with George Mason University and Maryland Department of the Environment Baltimore, Maryland 2000 U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY U.S. Department of the Interior BRUCE BABBITT, Secretary U.S. Geological Survey Charles G. Groat, Director The use of trade, product, or firm names in this report is for descriptive purposes only and does not imply endorsement by the U.S. Geological Survey. For additional information contact: District Chief U.S. Geological Survey 8987 Yellow Brick Road Baltimore, MD 21237 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286 Denver, CO 80225-0286 CONTENTS Abstract.........................................................................................................................................1 Introduction...................................................................................................................................1 Background ......................................................................................................................2 -

Characterization Upper Chester River Watershed in Kent County And
Characterization Of The Upper Chester River Watershed In Kent County and Queen Anne’s County, Maryland March 2005 In support of a Watershed Restoration Action Strategy for the Upper Chester River Watershed by Queen Anne’s County and Kent County Product of the Maryland Department of Natural Resources Watershed Services In Partnership With Queen Anne’s County and Kent County STATE OF MARYLAND Robert L. Ehrlich, Jr., Governor Michael S. Steele, Lt. Governor Maryland Department of Natural Resources C. Ronald Franks, Secretary Watershed Services Tawes State Office Building, 580 Taylor Avenue Annapolis, Maryland 21401-2397 Internet Address: http://www.dnr.maryland.gov Telephone Contact Information: Toll free in Maryland: 1-877-620-8DNR x8746, Out of state call: 410-260-8746 TTY users call via Maryland Relay The facilities and services of the Maryland Department of Natural Resources are available to all without regard to race, color, religion, sex, sexual orientation, age, national origin or physical or mental disability. This document is available in alternative format upon request from a qualified individual with disability A publication of the Maryland Coastal Zone Management Program, Department of Natural Resources pursuant to NOAA Award No. NA04NOS4190042. Financial as- sistance provided by the Coastal Zone Management Act of 1972, as amended, admin- istered by the Office of Ocean and Coastal Resource Management, National Oceanic and Atmospheric Administration (NOAA). Upper Chester River Watershed Characterization, March 2005 Publications Tracking -
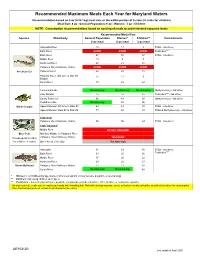
Recommended Maximum Fish Meals Each Year For
Recommended Maximum Meals Each Year for Maryland Waters Recommendation based on 8 oz (0.227 kg) meal size, or the edible portion of 9 crabs (4 crabs for children) Meal Size: 8 oz - General Population; 6 oz - Women; 3 oz - Children NOTE: Consumption recommendations based on spacing of meals to avoid elevated exposure levels Recommended Meals/Year Species Waterbody General PopulationWomen* Children** Contaminants 8 oz meal 6 oz meal 3 oz meal Anacostia River 15 11 8 PCBs - risk driver Back River AVOID AVOID AVOID Pesticides*** Bush River 47 35 27 PCBs - risk driver Middle River 13 9 7 Northeast River 27 21 16 Patapsco River/Baltimore Harbor AVOID AVOID AVOID American Eel Patuxent River 26 20 15 Potomac River (DC Line to MD 301 1511 9 Bridge) South River 37 28 22 Centennial Lake No Advisory No Advisory No Advisory Methylmercury - risk driver Lake Roland 12 12 12 Pesticides*** - risk driver Liberty Reservoir 96 48 48 Methylmercury - risk driver Tuckahoe Lake No Advisory 93 56 Black Crappie Upper Potomac: DC Line to Dam #3 64 49 38 PCBs - risk driver Upper Potomac: Dam #4 to Dam #5 77 58 45 PCBs & Methylmercury - risk driver Crab meat Patapsco River/Baltimore Harbor 96 96 24 PCBs - risk driver Crab "mustard" Middle River DO NOT CONSUME Blue Crab Mid Bay: Middle to Patapsco River (1 meal equals 9 crabs) Patapsco River/Baltimore Harbor "MUSTARD" (for children: 4 crabs ) Other Areas of the Bay Eat Sparingly Anacostia 51 39 30 PCBs - risk driver Back River 33 25 20 Pesticides*** Middle River 37 28 22 Northeast River 29 22 17 Brown Bullhead Patapsco River/Baltimore Harbor 17 13 10 South River No Advisory No Advisory 88 * Women = of childbearing age (women who are pregnant or may become pregnant, or are nursing) ** Children = all young children up to age 6 *** Pesticides = banned organochlorine pesticide compounds (include chlordane, DDT, dieldrin, or heptachlor epoxide) As a general rule, make sure to wash your hands after handling fish. -

Watersheds.Pdf
Watershed Code Watershed Name 02130705 Aberdeen Proving Ground 02140205 Anacostia River 02140502 Antietam Creek 02130102 Assawoman Bay 02130703 Atkisson Reservoir 02130101 Atlantic Ocean 02130604 Back Creek 02130901 Back River 02130903 Baltimore Harbor 02130207 Big Annemessex River 02130606 Big Elk Creek 02130803 Bird River 02130902 Bodkin Creek 02130602 Bohemia River 02140104 Breton Bay 02131108 Brighton Dam 02120205 Broad Creek 02130701 Bush River 02130704 Bynum Run 02140207 Cabin John Creek 05020204 Casselman River 02140305 Catoctin Creek 02130106 Chincoteague Bay 02130607 Christina River 02050301 Conewago Creek 02140504 Conococheague Creek 02120204 Conowingo Dam Susq R 02130507 Corsica River 05020203 Deep Creek Lake 02120202 Deer Creek 02130204 Dividing Creek 02140304 Double Pipe Creek 02130501 Eastern Bay 02141002 Evitts Creek 02140511 Fifteen Mile Creek 02130307 Fishing Bay 02130609 Furnace Bay 02141004 Georges Creek 02140107 Gilbert Swamp 02130801 Gunpowder River 02130905 Gwynns Falls 02130401 Honga River 02130103 Isle of Wight Bay 02130904 Jones Falls 02130511 Kent Island Bay 02130504 Kent Narrows 02120201 L Susquehanna River 02130506 Langford Creek 02130907 Liberty Reservoir 02140506 Licking Creek 02130402 Little Choptank 02140505 Little Conococheague 02130605 Little Elk Creek 02130804 Little Gunpowder Falls 02131105 Little Patuxent River 02140509 Little Tonoloway Creek 05020202 Little Youghiogheny R 02130805 Loch Raven Reservoir 02139998 Lower Chesapeake Bay 02130505 Lower Chester River 02130403 Lower Choptank 02130601 Lower -

Captain John Smith Chesapeake National Historic Trail Connecting
CAPTAIN JOHN SMITH CHESAPEAKE NATIONAL HISTORIC TRAIL CONNECTING TRAILS EVALUATION STUDY 410 Severn Avenue, Suite 405 Annapolis, MD 21403 CONTENTS Acknowledgments 2 Executive Summary 3 Statement of Study Findings 5 Introduction 9 Research Team Reports 10 Anacostia River 11 Chester River 15 Choptank River 19 Susquehanna River 23 Upper James River 27 Upper Nanticoke River 30 Appendix: Research Teams’ Executive Summaries and Bibliographies 34 Anacostia River 34 Chester River 37 Choptank River 40 Susquehanna River 44 Upper James River 54 Upper Nanticoke River 56 ACKNOWLEDGMENTS We are truly thankful to the research and project team, led by John S. Salmon, for the months of dedicated research, mapping, and analysis that led to the production of this important study. In all, more than 35 pro- fessionals, including professors and students representing six universities, American Indian representatives, consultants, public agency representatives, and community leaders contributed to this report. Each person brought an extraordinary depth of knowledge, keen insight and a personal devotion to the project. We are especially grateful for the generous financial support that we received from the following private foundations, organizations and corporate partners: The Morris & Gwendolyn Cafritz Foundation, The Clay- ton Fund, Inc., Colcom Foundation, The Conservation Fund, Lockheed Martin, the Richard King Mellon Foundation, The Merrill Foundation, the Pennsylvania Environmental Council, the Rauch Foundation, The Peter Jay Sharp Foundation, Verizon, Virginia Environmental Endowment and the Wallace Genetic Foundation. Without their support this project would simply not have been possible. Finally, we would like to extend a special thank you to the board of directors of the Chesapeake Conser- vancy, and to John Maounis, Superintendent of the National Park Service Chesapeake Bay Office, for their leadership and unwavering commitment to the Captain John Smith Chesapeake Trail. -

Currents the Yearly Journal of the Chester River Association Vol
CURRENTS THE YEARLY JOURNAL OF THE CHESTER RIVER ASSOCIATION VOL. 27 • 2017 Letter from the CONTENTS PRESIDENT Change. It comes with this time of 3 | Cover Story: Rethinking Ag year as the long days of summer give way to the vibrant colors of autumn. 4 | The Corsica? It’s Complicated The waterfowl are back, the rockfish have moved into the Chester, and the 7 | A Surge in SAV leaves have turned. At the Chester River Association office, change is also in the air. 8 | Tale of Two Creeks After long and careful deliberation through the spring and summer, CRA’s board approved a merger between CRA, Midshore Riverkeeper Conservancy, and the Sassafras River Association. The new organization will be called ShoreRivers. Evoking the image of the interconnectedness between land and water, ShoreRivers will be a powerful and unified voice for clean water on Maryland’s Eastern Shore and it will continue and expand the work of CRA in the Chester watershed. ShoreRivers will be based at the Eastern Shore Conservation Center in Easton, while maintaining the existing Chestertown and Georgetown offices of CRA and SRA. Our current Riverkeeper, Isabel Hardesty, will assume the role of Regional Director of the Chester and Sassafras programs, and Tim Trumbauer will become the next Chester Riverkeeper. The rest of our fantastic staff will continue to support efforts in these two northern watersheds. While there is much administrative work being done to ensure a smooth transition, the energy and excitement in the office are palpable. Our staff members were early and enthusiastic supporters of the merger, and are truly excited to bring the collective resources the merger represents into our local programming. -

Severn River Commission Presentation
69 Franklin Street Annapolis, MD 21401 410-216-9441 www.chesapeakelegal.org [email protected] OUR MISSION To use the law to protect and restore the Chesapeake Bay and its watershed by providing pro bono legal assistance through a network of volunteer attorneys. OUR VISION A clean, healthy Chesapeake Bay that can be enjoyed safely by humans, sustain viable fishing harvests, and support thriving and diverse aquatic and wildlife populations. HOW IT WORKS Intake and Evaluation Client CLA Attorney request for assistance connect with assistance Restoration and Protection Our Clients and Partners v Individuals v Ad-Hoc Coalitions v Community Groups v State and Local v Environmental Organizations Government v Conservation Organizations v Home Owners Associations v Coalitions CLA Clients 1000 Friends of Maryland Bedford County Citizen Choose Clean Water Federal Hill Residents Action Committee for Transit Group Coalition Food and Water Watch Agricultural Certainty Work Beechwood Hill Homeowners Choptank Riverkeeper Frederick County Citizens Group Association Citizens Against Plan for Group Allentown Residents for Berrywood Community Eva Mar Development Friends of Bryan Park Clean Air Association Citizens for a Better Charles Friends of Cabin John Creek Alliance for the Chesapeake Blue Water County Watershed Bay Baltimore/Baltimore Citizens to Protect Blu Point Friends of Hillmead Park Alliance to Restore Northwest Harbor Waterkeeper Citizens to Save Little Pimmit Friends of Jug Bay Creek BMAT Restoration Work Run Friends of Mc Millan Park -

Chester River and Choptank River Watershed Management Plan
Chester River and Choptank River Watershed Management Plan Final Plan November 2014 Prepared for: Department of Natural Resources and Environmental Control (DNREC) Prepared by: KCI Technologies, Inc. 1352 Marrows Road Suite 100 Newark, DE 19711 Chester River and Choptank River Watershed Management Plan Final Plan November 2014 Prepared for: Department of Natural Resources and Environmental Control (DNREC) Prepared by: KCI Technologies, Inc. 1352 Marrows Road Suite 100 Newark, DE 19711 KCI Job Order No. 17133560 Chester River and Choptank River Watershed Management Plan 2014 Table of Contents 1 Introduction ................................................................................................................... 5 1.1 Goals and Objectives ..................................................................................................................... 6 1.2 Regulatory and Programmatic Environment ................................................................................ 7 1.3 Watershed Priorities ..................................................................................................................... 8 2 Watershed Characteristics.............................................................................................. 8 2.1 Watershed Delineation and Planning Segments .......................................................................... 8 2.2 Chester and Choptank ................................................................................................................. 11 2.2.1 Chester River ...................................................................................................................... -

(Tmdls) Analysis for Chesapeake Bay Drainage Basin, Delaware
Total Maximum Daily Loads (TMDLs) Analysis for Chesapeake Bay Drainage Basin, Delaware: Chester River, Choptank River, Marshyhope Creek, Nanticoke River, Gum Branch, Gravelly Branch, Deep Creek, Broad Creek and Pocomoke River Watersheds Prepared by: Watershed Assessment Section Division of Water Resources Delaware Department of Natural Resources and Environmental Control 820 Silver Lake Boulevard, Suite 220 Dover, Delaware 19904 September 2006 _______________________________________Analysis of Chesapeake Drainage TMDLs, Delaware Table of Contents 1 1 Introduction/Background ......................................................................................... 1 1 1 Introduction/Background ......................................................................................... 1 2 Study Area....................................................................................................................3 2.1 Chester River Watershed ........................................................................................ 3 2.2 Choptank River Watershed..................................................................................... 5 2.3 Marshyhope Creek Watershed................................................................................ 7 2.4 Pocomoke River Watershed.................................................................................. 10 2.5 The Nanticoke River Basin................................................................................... 12 2.6 Designated Uses................................................................................................... -

Bay Grasses Backgrounder
Bay Grasses Backgrounder - Page 1 of 5 Upper Bay Zone In the Upper Bay Zone (21 segments extending south from the Susquehanna River to the Bay Bridge), underwater bay grasses increased 21 percent from 18,922 acres in 2007 to 22,954 acres in 2008. Ten of the 21 segments increased by at least 20 percent and by at least 12 acres from 2007 totals: 21%, Northern Chesapeake Bay Segment: 1,833 acres (2007) vs. 1,008 acres (2008) 21%, Northern Chesapeake Bay Segment 2: 11,725 acres (2007) vs. 14,194 acres (2008) 59%, Northeast River: 116 acres (2007) vs. 183 acres (2008) 70%, Upper Chesapeake Bay: 373 acres (2007) vs. 633 acres (2008) 50%, Sassafras River Segment: 1,368 acres (2007) vs. 551 acres (2008) 1204%, Sassafras River Segment 2: 5 acres (2007) vs. 52 acres (2008) 40%, Gunpowder River Segment 1: 558 acres (2007) vs. 783 acres (2008) 50%, Middle River: 551 acres (2007) vs. 828 acres (2008) 53%, Upper Central Chesapeake Bay: 124 acres (2007) vs. 188 acres (2008) 23%, Lower Chester River: 67 acres (2007) vs. 84 acres (2008) None of the 21 segments decreased by at least 20 percent and by at least 12 acres from 2007 totals. Two of the 21 segments remained unvegetated in 2008. Acres of SAV & Goal Attained Upper Chesapeake Bay Region Segment 2007 2008 Restoration Goal 2008 % of goal Northern Chesapeake Bay Segment 1 834 1007 754 134 Northern Chesapeake Bay Segment 2 11726 14194 12149 117 Northern Chesapeake Bay Total 12560 15202 12903 118 Northeast River 115 183 89 205 Elk River Segment 1 1590 1907 1844 103 Elk River Segment 2 391 440 190 232