Renewal Application of Kelly Cove Salmon Ltd. for AQ#1205 1. Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flood Frequency Analyses for New Brunswick Rivers Canadian Technical Report of Fisheries and Aquatic Sciences 2920
Flood Frequency Analyses for New Brunswick Rivers Aucoin, F., D. Caissie, N. El-Jabi and N. Turkkan Department of Fisheries and Oceans Gulf Region Oceans and Science Branch Diadromous Fish Section P.O. Box 5030, Moncton, NB, E1C 9B6 2011 Canadian Technical Report of Fisheries and Aquatic Sciences 2920 Canadian Technical Report of Fisheries and Aquatic Sciences Technical reports contain scientific and technical information that contributes to existing knowledge but which is not normally appropriate for primary literature. Technical reports are directed primarily toward a worldwide audience and have an international distribution. No restriction is placed on subject matter and the series reflects the broad interests and policies of Fisheries and Oceans, namely, fisheries and aquatic sciences. Technical reports may be cited as full publications. The correct citation appears above the abstract of each report. Each report is abstracted in the data base Aquatic Sciences and Fisheries Abstracts. Technical reports are produced regionally but are numbered nationally. Requests for individual reports will be filled by the issuing establishment listed on the front cover and title page. Numbers 1-456 in this series were issued as Technical Reports of the Fisheries Research Board of Canada. Numbers 457-714 were issued as Department of the Environment, Fisheries and Marine Service, Research and Development Directorate Technical Reports. Numbers 715-924 were issued as Department of Fisheries and Environment, Fisheries and Marine Service Technical Reports. The current series name was changed with report number 925. Rapport technique canadien des sciences halieutiques et aquatiques Les rapports techniques contiennent des renseignements scientifiques et techniques qui constituent une contribution aux connaissances actuelles, mais qui ne sont pas normalement appropriés pour la publication dans un journal scientifique. -

Industrial Park
VILLAGE OF PERTH-ANDOVER, N.B. Village of WH ET ERE P LS ME Perth-Andover EOPLE AND T RAI Perth-Andover Industrial Park "Home of the Best Power Rates in New Brunswick” CONTACT Mr. Dan Dionne Chief Administrative Officer Village of Perth-Andover 1131 West Riverside Drive Perth-Andover, New Brunswick E7H 5G5 Telephone: (506) 273-4959 Facsimile: (506) 273-4947 Email: [email protected] Website: www.perth-andover.com HISTORY OVERVIEW In 1991 the municipality established a 25 acre block of land for an industrial Perth-Andover is located on the Saint John River, 40 kilometres south of park. Several businesses have established themselves in the Industrial Grand Falls near the mouth of the Tobique River. Perth is located on the Park, and the municipality is currently expanding the park to accommodate east side of the river and Andover is located on the west side. The two future demand. Businesses wishing to establish in the park can expect the villages were amalgamated in 1966 and have a population service area in Mayor and Council to do whatever possible to assist them. Perth-Andover excess of 6,000 people. Nestled between the rolling hills of the upper river is ideally located for businesses looking for excellent access to the United valley, this picturesque village is often referred to as the "Gateway to the States and to Ontario and Quebec. Combine this with an excellent quality of Tobique". The Municipality is ten kilometres west of the U.S. border and life and you have one of the most attractive areas in the province for approximately 80 kilometres north of Woodstock and the entrance to locating new industry. -

Emotional Connection to Mactaquac Dam, River Valley Studied
Emotional connection to Mactaquac dam, river valley studied TARA CHISLETT Fredericton Daily Gleaner January 8, 2015 The Mactaquac Hydro Electric Dam near Fredericton. Photo: The Daily Gleaner archive How much do people in New Brunswick know about the Mactaquac dam and what it means for the future of energy in the province? That’s the question a team of social scientists from Dalhousie University, the University of New Brunswick and the University of Alberta are trying to answer as part of a larger research project on energy literacy, attitudes and values toward different energy options, and the effects of energy choices. The Mactaquac dam is expected to reach the end of its service life in 2030. NB Power has identified three options for the station: 1. refurbish; 2. rebuild; 3. decommission. Decommission would involve restoring the St. John River valley by draining the headpond above the dam. NB Power says it will be seeking input from experts, First Nations communities and New Brunswickers before selecting a preferred option in 2016. Kate Sherren, an assistant professor and academic program coordinator at Dalhousie University’s School for Resource and Environmental Studies, said the team received federal funding through a grant from the Social Sciences and Humanities Research Council in 2012, but work didn’t begin until summer 2013. The team is made up of made up of three principal investigators as well as several students. Along with the study of the Mactaquac dam, the project is also looking at sites in the Peace River, Alta., region and southern Ontario. The project isn’t designed to contribute directly to the decision about the dam, Sherren said. -

Septembre 2016 Utilisation Du Guide Des Points
MARCHANDISES GÉNÉRALES Points de Service Day & Ross Marchandises Générales Septembre 2016 Utilisation du guide des points Les points de parité et le guide des points publiés Tout tarif appliqué à un point associé à un point de parité ou basé sur celui-ci sera le même que le tarif du point de base. Par exemple, Dieppe, au Nouveau-Brunswick, PROV VILLE TERMINAL NOTE 1 NOTE 2 POINT DE BASE dont le terminal de livraison est Moncton (MTN), est associé à Moncton, au Nouveau-Brunswick. NB DIEPPE MTN MONCTON Par conséquent, tous les taux et frais de la grille tarifaire s’appliquant à Moncton, s’appliqueront également à Dieppe. Changements et exceptions Cette application ne peut pas être utilisée lorsque le tarif est publié pour un point autre qu’un point de parité tel que Dieppe. Par conséquent, le point précisé dans le tarif du client aura préséance, que Moncton soit également publié dans ce tarif ou non. Les tarifs des points ultérieurs de Corner Brook à Labrador sont saisonniers. Les tarifs aériens s’appliqueront en hiver si le service est oert, en absence du service de traversier, sinon les expéditions à destination de Labrador ne seront pas acceptées, à moins que le service puisse être assuré par le terminal de Québec. Les tarifs vers certains points du nord-ouest, de Nunavut, du Yukon et du Labrador peuvent changer en hiver et pendant la période de débâcle du printemps à cause des fermetures de route et de l’interruption du service de traversier. Le service aérien sera oert sur demande. Notes La note 1 du guide des points porte sur les jours de service supplémentaires qui pourraient s’avérer nécessaires. -

Up on Th'hill Down by the River by the Ocean Across the Field by the Word
Up on th’hill Down by the river By the ocean Across the field By the word of the Boognish Lordy lordy lord, I’m coming home -Gene & Dean Ween The Bristol-Shiktehawk bifaces and Early Woodland ceremonialism in the Middle St. John Valley, New Brunswick by Alexandre Pelletier-Michaud B.A., Université Laval, 2007 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Arts in the Graduate Academic Unit of Anthropology Supervisor: Susan E. Blair, Ph.D., Anthropology Examining Board: M. Gabriel Hrynick, Ph.D., Anthropology Gary K. Waite, Ph.D., History This thesis is accepted by the Dean of Graduate Studies THE UNIVERSITY OF NEW BRUNSWICK November, 2017 ©Alexandre Pelletier-Michaud, 2018 ABSTRACT In the Maritime Peninsula, the visibility of Early Woodland ceremonialism is limited to a few sites that have been associated with burial complexes defined elsewhere in the Northeast. The biface assemblage excavated in the 1930s from the Bristol- Shiktehawk site, in the middle St. John River Valley, has been assumed to be ceremonial but has never been the subject of a thorough professional analysis. I conduct such an analysis based on a technological approach. My results support the view that the assemblage likely dates to the Early Woodland period, by establishing connections which are rooted temporally in the region but extend geographically towards the Midwest. I question the compartmentalizing nomenclature which structures our understanding of regional variations in manifestations of ceremonialism, suggesting a more complex but fluid cultural landscape for the period around 3500 to 2000 B.P., and explore the limitations posed by the theoretical framework often applied to questions of ritual in archaeology. -

Life History Data on the Alewife and Blueback Herring of the Saint John River, New Brunswick ,1973
Canada. Fisheries and Marine Service. Maritimes Region. Resource Development Branch. DATA RECORD SERIES MAR/D 1411 Environment Canada Environnement Canada Fisheries Service des peches and Marine Service et des sciences de la mer Life History Data on the Alewife and Blueback Herring of the Saint John River, New Brunswick ,1973 by B.M. Jessop Data Record Series No. MAR/ D-77-2 Freshwater and Anadromous Division Resource Branch Maritimes Region LIFE HISTORY DATA ON THE ALEWIFE AND BLUEBACK HERRING OF THE SAINT JOHN RIVER, NEW BRUNSWICK, 1973 S.M. JESSOP FEBRUARY, 1973 DATA RECORD SERIES NO. MAR/D-77-2 FRESHWATER AND ANADROMOUS DIVISION RESOURCE BRANCH. FISHERIES AND MARINE SERVICE DEPARTMENT OF FISHERIES AND THE' ENVIRONMENT HALIFAX, NOVA SCOTIA iii CONTENTS LIST OF TABLES v LIST OF ILLUSTRATIONS ix INTRODUCTION 1 METHODS 1 RUN TIMING 2 RESULTS 2 DISCUSSION 3 ACKNOWLEDGEMENTS 47 REFERENCES 49 V LIST OF. TABLES TABLE 1. Mean fork lengths (mm), by sample date and location, for gaspereau from the Saint John River, 1973 5 TABLE 2. Mean fork lengths (mm), by sample date and location, for alewives from the Saint John River, 1973 6 TABLE 3. Length-frequency distributions of mature alewives, by sex and location, Saint John River, 1973 7 TABLE 4. Length-frequency distributions of immature alewives, by location, Saint John River, 1973 8 TABLE 5. Observed length-frequency of alewives, by age-groups, sexes and locations combined, Saint John River, 1973 8 TABLE 6. Mean fork lengths (mm) of alewives, by sex and age, by location, 1973. 1. Washademoak Lake 9 2. Grand Lake 9 3. -

24193667.Pdf
C S A S S C C S Canadian Science Advisory Secretariat Secrétariat canadien de consultation scientifique Research Document 2004/019 Document de recherche 2004/019 Not to be cited without Ne pas citer sans Permission of the authors * autorisation des auteurs * Assessments of Atlantic salmon Évaluations des stocks de saumon stocks in southwest New Brunswick, atlantique du sud-ouest du Nouveau an update to 2003 Brunswick : bilan jusqu’à 2003 R.A. Jones1, L. Anderson2, T. Goff2 1Department of Fisheries and Oceans Science Branch, Maritimes Region P.O. Box 5030 Moncton, NB E1C 9B6 2 Department of Fisheries and Oceans Science Branch, Maritimes Region Mactaquac Biodiversity Facility Kingsclear, NB E3E 2C6 * This series documents the scientific basis for the * La présente série documente les bases evaluation of fisheries resources in Canada. As scientifiques des évaluations des ressources such, it addresses the issues of the day in the halieutiques du Canada. Elle traite des time frames required and the documents it problèmes courants selon les échéanciers contains are not intended as definitive statements dictés. Les documents qu’elle contient ne on the subjects addressed but rather as progress doivent pas être considérés comme des énoncés reports on ongoing investigations. définitifs sur les sujets traités, mais plutôt comme des rapports d’étape sur les études en cours. Research documents are produced in the official Les documents de recherche sont publiés dans language in which they are provided to the la langue officielle utilisée dans le manuscrit Secretariat. envoyé au Secrétariat. This document is available on the Internet at: Ce document est disponible sur l’Internet à: http://www.dfo-mpo.gc.ca/csas/ ISSN 1499-3848 (Printed / Imprimé) © Her Majesty the Queen in Right of Canada, 2004 © Sa majesté la Reine, Chef du Canada, 2004 ABSTRACT Total one-sea-winter (1SW) (1,304) and multi-sea-winter (MSW) (752) returns destined for upstream of Mactaquac Dam on the Saint John River in 2003 were the second and third lowest, respectively, in 34 years of record. -

Annual Report 2018-19 Annual Report 2018-19
ANNUAL REPORT 2018-19 ANNUAL REPORT 2018-19 New Brunswick Energy & Utilities Board P.O. Box 5001 15 Market Square, Suite 1400 Saint John, New Brunswick E2L 4Y9 Telephone: (506) 658-2504 1-866-766-2782 Fax: (506) 643-7300 www.nbeub.ca This document has been printed on Enviro 100 paper: • Contains FSC certified 100% post-consumer fiber • Certified EcoLogo, Processed Chlorine Free and FSC Recycled • Manufactured using biogas energy TABLE OF CONTENTS 1 CHAIRPERSON’S MESSAGE 2 OVERVIEW 4 BOARD STRUCTURE 6 ELECTRICITY 8 RELIABILITY AND COMPLIANCE 9 MOTOR CARRIER 10 NATURAL GAS 11 PETROLEUM PRODUCTS 12 PIPELINE SAFETY 13 AUDITED FINANCIAL STATEMENTS CHAIRPERSON’S MESSAGE On behalf of the New Brunswick Energy and Utilities Board, and pursuant to Section 22 of the Energy and Utilities Board Act, I am pleased to present the Annual Report and Audited Financial Statements of the New Brunswick Energy and Utilities Board for the reporting period beginning April 1, 2018 and ending March 31, 2019. During this reporting period the Board received 36 applications and issued 28 decisions related to the five different sectors over which the Board has jurisdiction: Electricity, Natural Gas, Pipeline Safety, Petroleum Products and Motor Carrier. These decisions pertained to the setting of rates for Enbridge Gas New Brunswick and for the New Brunswick Power Corporation, the approval of pipeline permits and licenses, the approval of numerous electricity reliability standards and compliance issues and the granting of licenses for motor carriers. In addition, the Board has dealt with 37 formal inquiries from the general public during this reporting period. -
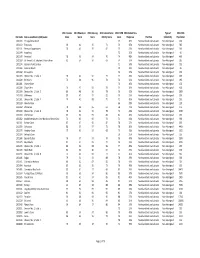
CSD Code Census Subdivision (CSD) Name 2011 Income Score
2011 Income 2011 Education 2011 Housing 2011 Labour Force 2011 CWB 2011 Global Non‐ Type of 2011 NHS CSD Code Census subdivision (CSD) name Score Score Score Activity Score Score Response Province Collectivity Population 1001105 Portugal Cove South 67 36% Newfoundland and Labrador Non‐Aboriginal 160 1001113 Trepassey 90 42 95 71 74 35% Newfoundland and Labrador Non‐Aboriginal 545 1001131 Renews‐Cappahayden 78 46 95 82 75 35% Newfoundland and Labrador Non‐Aboriginal 310 1001144 Aquaforte 72 31% Newfoundland and Labrador Non‐Aboriginal 90 1001149 Ferryland 78 53 94 70 74 48% Newfoundland and Labrador Non‐Aboriginal 465 1001169 St. Vincent's‐St. Stephen's‐Peter's River 81 54 94 69 74 37% Newfoundland and Labrador Non‐Aboriginal 315 1001174 Gaskiers‐Point La Haye 71 39% Newfoundland and Labrador Non‐Aboriginal 235 1001186 Admirals Beach 79 22% Newfoundland and Labrador Non‐Aboriginal 85 1001192 St. Joseph's 72 27% Newfoundland and Labrador Non‐Aboriginal 125 1001203 Division No. 1, Subd. X 76 44 91 77 72 45% Newfoundland and Labrador Non‐Aboriginal 495 1001228 St. Bride's 76 38 96 78 72 24% Newfoundland and Labrador Non‐Aboriginal 295 1001281 Chance Cove 74 40% Newfoundland and Labrador Non‐Aboriginal 120 1001289 Chapel Arm 79 47 92 78 74 38% Newfoundland and Labrador Non‐Aboriginal 405 1001304 Division No. 1, Subd. E 80 48 96 78 76 20% Newfoundland and Labrador Non‐Aboriginal 2990 1001308 Whiteway 80 50 93 82 76 25% Newfoundland and Labrador Non‐Aboriginal 255 1001321 Division No. 1, Subd. F 74 41 98 70 71 45% Newfoundland and Labrador Non‐Aboriginal 550 1001328 New Perlican 66 28% Newfoundland and Labrador Non‐Aboriginal 120 1001332 Winterton 78 38 95 61 68 41% Newfoundland and Labrador Non‐Aboriginal 475 1001339 Division No. -

Fishery Management in the Fish River Drainage
MAINE DEPARTMENT OF INLAND FISHERIES AND GAME FISHERY RESEARCH BULLETIN No. 6 Fishery Management in the Fish River Drainage by Kendall Warner Maine Department of Inland Fisheries and Game Augusta, Maine RONALD T. SPEERS, Commissioner Financed in part by Federal Aid to Fisheries Projects F-8-R, F-ll-R, Maine Published under A ppropriation # 7750 FOREWORD Your Inland Fisheries and Game Department is making continu ing biological studies of our lakes, rivers, and streams. The purpose of these studies is to evaluate existing and potential fisheries of our inland waters and to make recommendations to maintain the best possible management of our fisheries. As these studies on various river drainages are completed, the findings are presented to the citizens of our State. This report summarizes information collected on the fisheries of the waters in The Fish River drainage, Aroostook County, Maine. The field investigations were made by fishery biologists of the Fishery Research and Management Division of the Maine Depart ment of Inland Fisheries and Game over a period of 14 years, from 1950-1964. KENDALL WARNER, Regional Fishery Biologist Ashland, Maine •June, 1965 TABLE OF CONTENTS Page Forew ord ...................................................................................................... 2 Introduction ................................................................................................ 5 Description of the D ra in a g e ..................................................................... 6 Lake Management ..................................................................................... -

Passage Efficiency of the Tobique-Narrows Smolt By-Pass Facility: 2017-8 Results
Mactaquac Aquatic Ecosystem Study Report Series 2019-061 Passage Efficiency of the Tobique-Narrows Smolt By-Pass Facility: 2017-8 Results Kurt Samways, Mark Gautreau, and R. Allen Curry March 31, 2019 MAES Report Series 2019-061 Correct citation for this publication: Samways, K., M. Gautreau, and R. Allen Curry. 2019. Passage Efficiency of the Tobique- Narrows Smolt By-Pass Facility: 2017-8 Results. Mactaquac Aquatic Ecosystem Study Report Series 2019-061. Canadian Rivers Institute, University of New Brunswick, 27p. DISCLAIMER Intended Use and Technical Limitations of this report, “Passage Efficiency of the Tobique-Narrows Smolt By-Pass Facility”. This report describes the efficiency of the smolt by-pass facility at the Tobique-Narrows hydropower generating. The CRI does not assume liability for any use of the included information outside the stated scope. ii | Page MAES Report Series 2019-061 Table of Contents 1. Introduction ................................................................................................................................ 4 2. Methodology ............................................................................................................................... 5 2.1 Tagging .................................................................................................................................................. 6 2.2 Tracking ................................................................................................................................................ 8 3. Results ....................................................................................................................................... -

The Royal Gazette Index 2009
The Royal Gazette Gazette royale Fredericton Fredericton New Brunswick Nouveau-Brunswick ISSN 0703-8623 Index 2009 Volume 167 Table of Contents / Table des matières Page Proclamations . 2 Orders in Council / Décrets en conseil . 2 Legislative Assembly / Assemblée législative. 7 Elections NB / Élections Nouveau-Brunswick . 7 Departmental Notices / Avis ministériels . 8 NB Energy and Utilities Board / Commission de l’énergie et des services publics du N.-B. 13 New Brunswick Securities Commission / Commission des valeurs mobilières du Nouveau-Brunswick . 13 Notices Under Various Acts and General Notices / Avis en vertu de diverses lois et avis divers . 14 Sheriff’s Sales / Ventes par exécution forcée . 15 Notices of Sale / Avis de vente . 15 Regulations / Règlements . 17 Corporate Affairs Notices / Avis relatifs aux entreprises . 20 Business Corporations Act / Loi sur les corporations commerciales . 20 Companies Act / Loi sur les compagnies . 54 Partnerships and Business Names Registration Act / Loi sur l’enregistrement des sociétés en nom collectif et des appellations commerciales . 56 Limited Partnership Act / Loi sur les sociétés en commandite . 85 2009 Index Proclamations Workplace Health, Safety and Compensation Act, An Act to Amend the / Commission de la santé, de la sécurité et de l’indemnisation des accidents Acts / Lois au travail, Loi modifiant la Loi sur la—OIC/DC 2009-56—p. 463 (March 25 mars) Agricultural Development Board, the New Brunswick Fisheries and Aquaculture Development Board and the Transfer of Responsibility for Financial Assistance Programs, An Act Respecting the / Commission de l’aménagement agricole, le Conseil de développement des pêches et de Proclamations l’aquaculture du Nouveau-Brunswick et le transfert des responsabilités au General / Divers titre des programmes d’aide financière, Loi concernant la—OIC/DC 2009-351—p.