Overview of Algorithms to Be Used for Detecting COPD, COPD Exacerbations and Pneumonia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

Betamethasone
Betamethasone Background Betamethasone is a potent, long-acting, synthetic glucocorticoid widely used in equine veterinary medicine as a steroidal anti-inflammatory.1 It is often administered intra-articularly for control of pain associated with inflammation and osteoarthritis.2 Betamethasone is a prescription medication and can only be dispensed from or upon the request of a http://en.wikipedia.org/wiki/Betamethasone#/media/File:Betamethasone veterinarian. It is commercially available .png in a variety of formulations including BetaVet™, BetaVet Soluspan Suspension® and Betasone Aqueous Suspension™.3 Betamethasone can be used intra-articularly, intramuscularly, by inhalation, and topically.4 When administered intra-articularly, it is often combined with other substances such as hyaluronan.5 Intra-articular and intramuscular dosages range widely based upon articular space, medication combination protocol, and practitioner preference. Betamethasone is a glucocorticoid receptor agonist which binds to various glucocorticoid receptors setting off a sequence of events affecting gene transcription and the synthesis of proteins. These mechanisms of action include: • Potential alteration of the G protein-coupled receptors to interfere with intracellular signal transduction pathways • Enhanced transcription in many genes, especially those involving suppression of inflammation. • Inhibition of gene transcription – including those that encode pro-inflammatory substances. The last two of these are considered genomic effects. This type of corticosteroid effect usually occurs within hours to days after administration. The genomic effects persist after the concentrations of the synthetic corticosteroid in plasma are no longer detectable, as evidenced by persistent suppression of the normal production of hydrocortisone following synthetic corticosteroid administration.6 When used judiciously, corticosteroids can be beneficial to the horse. -

Chronic Obstructive Lung Disease
Chronic Obstructive Lung Disease Amita Vasoya, DO FACOI FCCP FAASM Christiana Care Pulmonary Associates Clinical Assistant Professor of Medicine Sidney Kimmel Medical College of Thomas Jefferson University Rowan University School of Osteopathic Medicine ACOI Board Review 2019 Disclosures No Disclosures Obstructive Lung Diseases COPD Chronic ◦ Chronic Bronchitis Bronchitis Emphysema ◦ Emphysema Asthma Other ◦ Bronchiectasis Asthma ◦ Bronchiolitis ◦ Cystic Fibrosis ◦ Alpha 1 anti-trypsin deficiency Inter-relationship: Inflammation and Bronchial Hyperreactivity ATS GOLD CHEST 2002; 121: 121S-126S COPD THIRD leading cause of death worldwide It is the only leading cause of death whose prevalence is increasing! http://www.who.int/mediacentre/factsheets COPD Risk Factors Cigarette smoking Occupational exposures ◦ Silica, formaldehyde, toluene, nickel, cadmium, cotton, dust, etc Air pollution Biomass fuel Hyperresponsive airway Asthma Genetic factors Pathogenesis of COPD ATS Pulmonary Board Review 2015 Inflammatory Mediators: COPD ATS Pulmonary Board Review 2015 INFLAMMATION Small Airway Disease Parenchyma destruction Airway inflammation Loss of alveolar attachments Airway remodeling Decreased elastic recoil AIRFLOW LIMITATION ATS Pulmonary Board Review 2015 COPD Phenotypes Non-exacerbator Exacerbator with emphysema Exacerbator with chronic bronchitis Frequent exacerbator Alpha 1 Antitrypsin deficiency ACOS BCOS www.eclipse-copd.com, Lange P. Int J COPD 2016. 11: 3-12 Hurst JR. NEJM 2010. 363: 1128-38 Morphologic Types of -

Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy Using Machine Learning
Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy using Machine Learning Kate Wang et al. Supplemental Table S1. Drugs selected by Pharmacophore-based, ML-based and DL- based search in the FDA-approved drugs database Pharmacophore WEKA TF 1-Palmitoyl-2-oleoyl-sn-glycero-3- 5-O-phosphono-alpha-D- (phospho-rac-(1-glycerol)) ribofuranosyl diphosphate Acarbose Amikacin Acetylcarnitine Acetarsol Arbutamine Acetylcholine Adenosine Aldehydo-N-Acetyl-D- Benserazide Acyclovir Glucosamine Bisoprolol Adefovir dipivoxil Alendronic acid Brivudine Alfentanil Alginic acid Cefamandole Alitretinoin alpha-Arbutin Cefdinir Azithromycin Amikacin Cefixime Balsalazide Amiloride Cefonicid Bethanechol Arbutin Ceforanide Bicalutamide Ascorbic acid calcium salt Cefotetan Calcium glubionate Auranofin Ceftibuten Cangrelor Azacitidine Ceftolozane Capecitabine Benserazide Cerivastatin Carbamoylcholine Besifloxacin Chlortetracycline Carisoprodol beta-L-fructofuranose Cilastatin Chlorobutanol Bictegravir Citicoline Cidofovir Bismuth subgallate Cladribine Clodronic acid Bleomycin Clarithromycin Colistimethate Bortezomib Clindamycin Cyclandelate Bromotheophylline Clofarabine Dexpanthenol Calcium threonate Cromoglicic acid Edoxudine Capecitabine Demeclocycline Elbasvir Capreomycin Diaminopropanol tetraacetic acid Erdosteine Carbidopa Diazolidinylurea Ethchlorvynol Carbocisteine Dibekacin Ethinamate Carboplatin Dinoprostone Famotidine Cefotetan Dipyridamole Fidaxomicin Chlormerodrin Doripenem Flavin adenine dinucleotide -

BREO • Do Not Use in Combination with an Additional Medicine Containing a ELLIPTA Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION treat acute symptoms. (5.2) These highlights do not include all the information needed to use BREO • Do not use in combination with an additional medicine containing a ELLIPTA safely and effectively. See full prescribing information for LABA because of risk of overdose. (5.3) BREO ELLIPTA. • Candida albicans infection of the mouth and pharynx may occur. Monitor patients periodically. Advise the patient to rinse his/her mouth with water BREO ELLIPTA 100/25 (fluticasone furoate 100 mcg and vilanterol without swallowing after inhalation to help reduce the risk. (5.4) 25 mcg inhalation powder), for oral inhalation • Increased risk of pneumonia in patients with COPD. Monitor patients for BREO ELLIPTA 200/25 (fluticasone furoate 200 mcg and vilanterol signs and symptoms of pneumonia. (5.5) 25 mcg inhalation powder), for oral inhalation • Potential worsening of infections (e.g., existing tuberculosis; fungal, Initial U.S. Approval: 2013 bacterial, viral, or parasitic infections; ocular herpes simplex). Use with caution in patients with these infections. More serious or even fatal course WARNING: ASTHMA-RELATED DEATH of chickenpox or measles can occur in susceptible patients. (5.6) See full prescribing information for complete boxed warning. • Risk of impaired adrenal function when transferring from systemic • Long-acting beta2-adrenergic agonists (LABA), such as vilanterol, corticosteroids. Taper patients slowly from systemic corticosteroids if increase the risk of asthma-related death. A placebo-controlled trial transferring to BREO ELLIPTA. (5.7) with another LABA (salmeterol) showed an increase in • Hypercorticism and adrenal suppression may occur with very high asthma-related deaths. This finding with salmeterol is considered a dosages or at the regular dosage in susceptible individuals. -

Fluticasone Furoate/Vilanterol 92/22 Μg Once-A-Day Vs Beclomethasone Dipropionate/Formoterol 100/6 Μg B.I.D
Open Access Journal of Pulmonology and Respiratory Research Research Article Fluticasone furoate/Vilanterol 92/22 μg once-a-day vs Beclomethasone dipropionate/Formoterol 100/6 μg b.i.d. in asthma patients: a 12-week pilot study Claudio Terzano* and Francesca Oriolo Respiratory Diseases Unit and School of Specialization in Respiratory Diseases Policlinico Umberto I, “Sapienza” University of Rome, Italy *Address for Correspondence: Dr Claudio Abstract Terzano, Respiratory Diseases Unit and School of Specialization in Respiratory Two of the most recent LABA/ICS combinations for treatment of persistent asthma are Fluticasone furoate/ Diseases Policlinico Umberto I, “Sapienza” Vilanterol 92/22 μg (Ellipta) and Beclomethasone dipropionate/Formoterol 100/6 μg (Nexthaler). University of Rome, Italy, Tel: 0649979051; Fax: 06499790675; Email: Objective: To compare once-daily Fluticasone/ Vilanterol combination with twice daily Beclomethasone/ [email protected] Formoterol association in moderate asthma, in terms of quality of life and lung function. Submitted: 08 September 2017 Methods: Fourty patients with moderate asthma treated with Beclomethasone/Formoterol 100/6 μg or Approved: 26 September 2017 Fluticasone/Vilanterol 92/22 μg. We revalued patients in terms of lung function and Asthma Control Test, at 4, 8 Published: 27 September 2017 and 12 weeks to assess any differences between the two groups. After 4 weeks, thirty-one of the fourty patients were evaluated in terms of respiratory function at predetermined time intervals. Copyright: 2017 Terzano C, et al. This is an open access article distributed under the Result: In patients treated with beclomethasone/formoterol FEV1 presented a mean value of 78% at the Creative Commons Attribution License, which third visit and of 79.1% during the fi nal check, compared with 74.5% and to 75.8% in patients in treatment permits unrestricted use, distribution, and with fl uticasone/vilanterol (p 0.01). -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME TRELEGY ELLIPTA 100/62.5/25 fluticasone furoate (100 micrograms)/umeclidinium (as bromide) (62.5 micrograms)/vilanterol (as trifenatate) (25 micrograms), powder for inhalation 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each delivered dose (the dose leaving the mouthpiece of the inhaler) contains 92 micrograms fluticasone furoate, 55 micrograms umeclidinium (equivalent to 65 micrograms umeclidinium [as bromide]) and 22 micrograms vilanterol (as trifenatate). This corresponds to a pre-dispensed dose of 100 micrograms fluticasone furoate, 62.5 micrograms umeclidinium (equivalent to 74.2 micrograms umeclidinium bromide) and 25 micrograms vilanterol (as trifenatate). Excipient with known effect: Each delivered dose contains approximately 25 milligrams of lactose (as monohydrate). For the full list of excipients, see Section 6.1 List of excipients. 3. PHARMACEUTICAL FORM Powder for inhalation. White powder in a light grey inhaler (Ellipta) with a beige mouthpiece cover and a dose counter. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications TRELEGY ELLIPTA is indicated for the maintenance treatment of adults with moderate to severe chronic obstructive pulmonary disease (COPD) who require treatment with a long- acting muscarinic receptor antagonist (LAMA) + long-acting beta2-receptor agonist (LABA) + inhaled corticosteroid (ICS). TRELEGY ELLIPTA should not be used for the initiation of COPD treatment. 4.2. Dose and method of administration Patients can be changed from their existing inhalers to TRELEGY ELLIPTA at the next dose. However it is important that patients do not take other LABA or LAMA or ICS while taking TRELEGY ELLIPTA. A stepwise approach to the management of COPD is recommended, including the cessation of smoking and a pulmonary rehabilitation program. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -
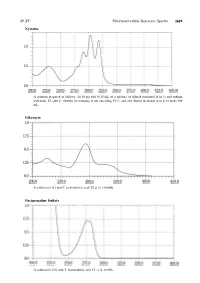
Nystatin Ofloxacin Orciprenaline Sulfate
JP XV Ultraviolet-visible Reference Spectra 1619 Nystatin A solution prepared as follows: To 10 mg add 50.25 mL of a mixture of diluted methanol (4 in 5) and sodium hydroxide TS (200:1), dissolve by warming at not exceeding 509C,andadddilutedmethanol(4in5)tomake500 mL. Ofloxacin Asolutionin0.1mol/LhydrochloricacidTS(1in150,000) Orciprenaline Sulfate A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) 1620 Ultraviolet-visible Reference Spectra JP XV Oxazolam A solution in ethanol (95) (1 in 100,000) Oxethazaine A solution in ethanol (95) (1 in 2500) Oxybuprocaine Hydrochloride An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1621 Oxycodone Hydrochloride Hydrate An aqueous solution (1 in 10,000) Oxymetholone A solution prepared as follows: To 5 mL of a solution in methanol (1 in 5000) add 5 mL of sodium hydroxide-methanol TS and methanol to make 50 mL. Oxytetracycline Hydrochloride Asolutionin0.1mol/LhydrochloricacidTS(1in50,000) 1622 Ultraviolet-visible Reference Spectra JP XV Oxytocin An aqueous solution (1 in 2000) Penbutolol Sulfate A solution in methanol (1 in 10,000) Pentazocine A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) JP XV Ultraviolet-visible Reference Spectra 1623 Peplomycin Sulfate Asolutionpreparedasfollows:To4mgadd5mL of copper (II) sulfate TS, and dissolve in water to make 100 mL. Perphenazine 1 Asolutionin0.1mol/LhydrochloricacidTS(1in200,000) Perphenazine 2 A solution obtained by adding 10 mL of water to 10 mL of the solution for Perphenazine 1 1624 Ultraviolet-visible -

FDA Briefing Document Pulmonary-Allergy Drugs Advisory Committee Meeting
FDA Briefing Document Pulmonary-Allergy Drugs Advisory Committee Meeting August 31, 2020 sNDA 209482: fluticasone furoate/umeclidinium/vilanterol fixed dose combination to reduce all-cause mortality in patients with chronic obstructive pulmonary disease NDA209482/S-0008 PADAC Clinical and Statistical Briefing Document Fluticasone furoate/umeclidinium/vilanterol fixed dose combination for all-cause mortality DISCLAIMER STATEMENT The attached package contains background information prepared by the Food and Drug Administration (FDA) for the panel members of the advisory committee. The FDA background package often contains assessments and/or conclusions and recommendations written by individual FDA reviewers. Such conclusions and recommendations do not necessarily represent the final position of the individual reviewers, nor do they necessarily represent the final position of the Review Division or Office. We have brought the supplemental New Drug Application (sNDA) 209482, for fluticasone furoate/umeclidinium/vilanterol, as an inhaled fixed dose combination, for the reduction in all-cause mortality in patients with COPD, to this Advisory Committee in order to gain the Committee’s insights and opinions, and the background package may not include all issues relevant to the final regulatory recommendation and instead is intended to focus on issues identified by the Agency for discussion by the advisory committee. The FDA will not issue a final determination on the issues at hand until input from the advisory committee process has been considered -

Clenbuterol Elisa Kit Instructions Product #101219 & 101216 Forensic Use Only
Neogen Corporation 944 Nandino Blvd., Lexington KY 40511 USA 800/477-8201 USA/Canada | 859/254-1221 Fax: 859/255-5532 | E-mail: [email protected] | Web: www.neogen.com/Toxicology CLENBUTEROL ELISA KIT INSTRUCTIONS PRODUCT #101219 & 101216 FORENSIC USE ONLY INTENDED USE: For the determination of trace quantities of Clenbuterol and/or other metabolites in human urine, blood, oral fluid. DESCRIPTION Neogen Corporation’s Clenbuterol ELISA (Enzyme-Linked ImmunoSorbent Assay) test kit is a qualitative one-step kit designed for use as a screening device for the detection of drugs and/or their metabolites. The kit was designed for screening purposes and is intended for forensic use only. It is recommended that all suspect samples be confirmed by a quantitative method such as gas chromatography/mass spectrometry (GC/MS). ASSAY PRINCIPLES Neogen Corporation’s test kit operates on the basis of competition between the drug or its metabolite in the sample and the drug- enzyme conjugate for a limited number of antibody binding sites. First, the sample or control is added to the microplate. Next, the diluted drug-enzyme conjugate is added and the mixture is incubated at room temperature. During this incubation, the drug in the sample or the drug-enzyme conjugate binds to antibody immobilized in the microplate wells. After incubation, the plate is washed 3 times to remove any unbound sample or drug-enzyme conjugate. The presence of bound drug-enzyme conjugate is recognized by the addition of K-Blue® Substrate (TMB). After a 30 minute substrate incubation, the reaction is halted with the addition of Red Stop Solution. -

Therapeutic Drug Class
BUREAU FOR MEDICAL SERVICES EFFECTIVE WEST VIRGINIA MEDICAID PREFERRED DRUG LIST WITH PRIOR AUTHORIZATION CRITERIA 07/01/2018 This is not an all-inclusive list of available covered drugs and includes only Version 2018.3e managed categories. Refer to cover page for complete list of rules governing this PDL. • Prior authorization for a non-preferred agent in any class will be given only if there has been a trial of the preferred brand/generic equivalent or preferred formulation of the active ingredient, at a therapeutic dose, that resulted in a partial response with a documented intolerance. • Prior authorization of a non-preferred isomer, pro-drug, or metabolite will be considered with a trial of a preferred parent drug of the same chemical entity, at a therapeutic dose, that resulted in a partial response with documented intolerance or a previous trial and therapy failure, at a therapeutic dose, with a preferred drug of a different chemical entity indicated to treat the submitted diagnosis. (The required trial may be overridden when documented evidence is provided that the use of these preferred agent(s) would be medically contraindicated.) • Unless otherwise specified, the listing of a particular brand or generic name includes all legend forms of that drug. OTC drugs are not covered unless specified. • PA criteria for non-preferred agents apply in addition to general Drug Utilization Review policy that is in effect for the entire pharmacy program, including, but not limited to, appropriate dosing, duplication of therapy, etc. • The use of pharmaceutical samples will not be considered when evaluating the members’ medical condition or prior prescription history for drugs that require prior authorization.