Pilbara, Western Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Agamid Lizards of the Genera Caimanops, Physignathus and Diporiphora in Western Australia and Northern Territory
Rec. West. Aust. Mus., 1974, 3 (2) AGAMID LIZARDS OF THE GENERA CAIMANOPS, PHYSIGNATHUS AND DIPORIPHORA IN WESTERN AUSTRALIA AND NORTHERN TERRITORY G.M. STORR [Received 11 February 1974. Accepted 15 February 1974] ABSTRACT Caimanopsgen. novo is proposed for Diporiphora amphiboluroides Lucas & Frost. The following species and subspecies ofPhysignathus and Diporiphora are studied: P. longirostris (Boulenger), P. temporalis (Giinther), P. g. gilberti (Gray), P. g. centralis Loveridge, D. convergens nov., D. a. albilabris nov., D. a. sobria nov., D. b. bennettii (GraY), D. b. arnhemica nov., D. magna nov., D. lalliae nov., D. reginae Glauert, D. winneckei Lucas & Frost, D. b. bilineata Gray, D. b. margaretae nov., and D. superba novo INTRODUCTION Recent collections have made it increasingly clear that there are many more species of Diporiphora in the far north of Western Australia than previously believed. The main purpose of this paper is to define these additional species of Diporiphora. Because juvenile Physignathus have often been mistaken for Diporiphora, that genus has been included in this study, and so too has Caimanops gen. nov., whose single species was long placed in Diporiphora. Generally Western Australian species of reptiles seldom extend further east than about longitude 140o E. Brief study of Queensland material showed that Diporiphora and Physignathus were not exceptional in this respect and that most, if not all, specimens belonged to different species or subspecies. It therefore seemed unnecessary to include the Eastern States species in this account of the Western species. The three species of Physignathus and single species of Caimanops are strongly characterized, and their identification should present students with no problems. -

Introduction of an Alien Fish Species in the Pilbara Region of Western
RECORDS OF THE WESTERN AUSTRALIAN MUSEUM 33 108–114 (2018) DOI: 10.18195/issn.0312-3162.33(1).2018.108-114 Introduction of an alien fsh species in the Pilbara region of Western Australia Dean C. Thorburn1, James J. Keleher1 and Simon G. Longbottom1 1 Indo-Pacifc Environmental, PO Box 191, Duncraig East, Western Australia 6023, Australia. * Corresponding author: [email protected] ABSTRACT – Until recently rivers of the Pilbara region of north Western Australia were considered to be free of introduced fsh species. However, a survey of aquatic fauna of the Fortescue River conducted in March 2017 resulted in the capture of 19 Poecilia latipinna (Sailfn Molly) throughout a 25 km section of the upper catchment. This represented the frst record of an alien fsh species in the Pilbara region and the most northern record in Western Australia. Based on the size of the individuals captured, the distribution over which they were recorded and the fact that the largest female was mature, P. latipinna appeared to be breeding. While P. latipinna was unlikely to physically threaten native fsh species in the upper reaches of the Fortescue River, potential spatial and dietary competition may exist if it reaches downstream waters where native fsh diversity is higher and dietary overlap is likely. As P. latipinna has the potential to affect macroinvertebrate communities, some risk may also exist to the macroinvertebrate community of the Fortescue Marsh, which is located immediately downstream, and which is valued for its numerous short range endemic aquatic invertebrates. The current fnding indicated that despite the relative isolation of the river and presence of a low human population, this remoteness does not mean the river is safe from the potential impact of species introductions. -

Port Hedland AREA PLANNING STUDY
Port Hedland AREA PLANNING STUDY Published by the Western Australian Planning Commission Final September 2003 Disclaimer This document has been published by the Western Australian Planning Commission. Any representation, statement, opinion or advice expressed or implied in this publication is made in good faith and on the basis that the Government, its employees and agents are not liable for any damage or loss whatsoever which may occur as a result of action taken or not taken (as the case may be) in respect of any representation, statement, opinion or advice referred to herein. Professional advice should be obtained before applying the information contained in this document to particular circumstances. © State of Western Australia Published by the Western Australian Planning Commission Albert Facey House 469 Wellington Street Perth, Western Australia 6000 Published September 2003 ISBN 0 7309 9330 2 Internet: http://www.wapc.wa.gov.au e-mail: [email protected] Phone: (08) 9264 7777 Fax: (08) 9264 7566 TTY: (08) 9264 7535 Infoline: 1800 626 477 Copies of this document are available in alternative formats on application to the Disability Services Co-ordinator. Western Australian Planning Commission owns all photography in this document unless otherwise stated. Port Hedland AREA PLANNING STUDY Foreword Port Hedland is one of the Pilbara’s most historic and colourful towns. The townsite as we know it was established by European settlers in 1896 as a service centre for the pastoral, goldmining and pearling industries, although the area has been home to Aboriginal people for many thousands of years. In the 1960s Port Hedland experienced a major growth period, as a direct result of the emerging iron ore industry. -

Terrific Walk Trails
TERRIFIC WALK TRAILS Walk trails are such a fantastic way to GERALDTON - BLUFF POINT(2KM) explore your surrounds. Check out The trail starts in Rundle Park at St Georges Beach, some of the best on offer locally. just off Kempton Street. Follow the markers to the site of the Bluff Point Lighthouse, which was GERALDTON - WEST END (2KM) officially lit on 23 October 1876 and the first Start the West End Trail outside the Mid West Port Lighthouse Keeper’s Cottage is on this trail. The Authority building on Francis Street and follow Lighthouse Keeper’s Cottage remains today. the markers. Much of the early development of You can also visit the area where the Bluff Point Geraldton took place within 500 metres of the Railway Junction connected the Perth to Geraldton start of this trail. The trail looks at the history of line with the Northampton line in 1886. Bluff Point the people who came to Champion Bay. Several was considered to be on the outskirts of town and a World War II military command posts were in the school, churches and shops were built for the local West End. Learn how after the war the West End community. went through a dramatic change as the harbour Start: Kempton St, Bluff Point expanded and the fishing industry developed. Start: 298 Marine Tce, Geraldton GERALDTON - VICTORIA HOSPITAL (650M) This trail starts outside the Bill Sewell Complex GERALDTON - MARINE TCE (2KM) on the corner of Chapman Road and Bayly Street. The Marine Terrace Trail begins on the Geraldton Follow the markers on a path back through time; Foreshore outside Dome Cafe. -
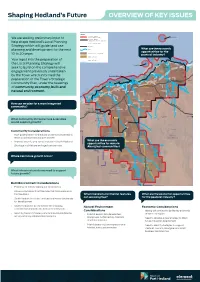
We Are Seeking Preliminary Input to Help Shape
Shaping Hedland’s Future OVERVIEW OF KEY ISSUES LEGEND Town of Port Hedland Redout Island Local Government Boundary We are seeking preliminary input to Town of Port Hedland Town Planning Scheme No. 5 Boundary help shape Hedland’s Local Planning Local Government Boundary Strategy which will guide land use Key Roads What are the economic planning and development for the next Rivers opportunities for the Pastoral Lease / Station Boundary 10 to 20 years. pastoral industry?North Turtle Island Indigenous Reserve Your input into the preparation of Indigenous Campsite the Local Planning Strategy will Information Turtle Island Information seek to build on the comprehensive Ripon Island Information De Grey engagement previously undertaken Information Ridley River by the Town which informed the Information asdasd Port Hedland Information De Grey River preparation of the Town’s Strategic NORT asdasd Tjalka Boorda T HERN HIG Y Pardoo GREA HWA Information Marta Marta Community Plan, under the headings asdasd Punju Njamal Information De Grey asdasd of community, economy, built and Tjalka Wara Jinparinya Information asdasd Strelley Shaw River natural environment. Information Pippingara asdasd South West Creek Shelley River Information asdasd Burrup Information Boodarie Point Samson asdasd Peawah River Information Cossack asdasd M How can we plan for a more integrated Balla Balla Mundabullangana ARB Karratha Information Turner River LE BAR asdasd Wallareenya RO community? Poverty Yule River Roebourne Information A Whim Creek Creek D asdasd ST COAS TAL -

Minister Declines Cultural Heritage Protection Conversation with Aboriginal People
Minister declines Cultural Heritage protection conversation with Aboriginal people MEDIA RELEASE: 23 August 2021 On the eve of the 7th Annual On-Country Bush Meeting at the Yule River Meeting Place, it is with disappointment that YMAC shares the news that the Minister for Aboriginal Affairs has declined the opportunity to attend the important event. The Yule River meeting, which is where Aboriginal people will discuss their concerns about cultural heritage protection, is being held on Wednesday 25 and Thursday 26 August. The focus of discussions will be on the Aboriginal Cultural Heritage Bill, which Minister Stephen Dawson intends to introduce to parliament this year. During the State Government’s 18 August presentation on its latest amendments to the Bill limited information was provided to show how - in practice - heritage will be able to be protected by the owners of Australia’s Cultural Heritage in Western Australia. YMAC CEO Mr Simon Hawkins said the Minister’s no-show is a missed opportunity to hear directly from the community. “The Labor Government consistently states that agreement-making is at the heart of the Aboriginal Cultural Heritage Bill, but the Minister’s non-attendance flies in the face of those claims.” “It is disappointing that the Minister, who is also the Member for the Mining and Pastoral Region, has not seen fit to send a representative on his behalf to what is one of the most important annual events for the Aboriginal and Torres Strait Islander peoples of the Pilbara.” The Minister has attended previous Yule River meetings and has made a commitment to co- designing the associated regulations for the new heritage laws with Aboriginal people. -

Port Hedland Regional Water Supply
DE GREY RIVER WATER RESERVE WATER SOURCE PROTECTION PLAN Port Hedland Regional Water Supply WATER RESOURCE PROTECTION SERIES WATER AND RIVERS COMMISSION REPORT WRP 24 2000 WATER AND RIVERS COMMISSION HYATT CENTRE 3 PLAIN STREET EAST PERTH WESTERN AUSTRALIA 6004 TELEPHONE (08) 9278 0300 FACSIMILE (08) 9278 0301 WEBSITE: http://www.wrc.wa.gov.au We welcome your feedback A publication feedback form can be found at the back of this publication, or online at http://www.wrc.wa.gov.au/public/feedback/ (Cover Photograph: The De Grey River) _______________________________________________________________________________________________ DE GREY RIVER WATER RESERVE WATER SOURCE PROTECTION PLAN Port Hedland Regional Water Supply Water and Rivers Commission Policy and Planning Division WATER AND RIVERS COMMISSION WATER RESOURCE PROTECTION SERIES REPORT NO. WRP 24 2000 i _______________________________________________________________________________________________ Acknowledgments Contribution Personnel Title Organisation Supervision Ross Sheridan Program Manager, Protection Planning Water and Rivers Commission Hydrogeology / Hydrology Angus Davidson Supervising Hydrogeologist, Groundwater Water and Rivers Commission Resources Appraisal Report Preparation John Bush Consultant Environmental Scientist Auswest Consultant Ecology Services Dot Colman Water Resources Officer Water and Rivers Commission Rueben Taylor Water Resources Planner Water and Rivers Commission Rachael Miller Environmental Officer Water and Rivers Commission Drafting Nigel Atkinson Contractor McErry Digital Mapping For more information contact: Program Manager, Protection Planning Water Quality Protection Branch Water and Rivers Commission 3 Plain Street EAST PERTH WA 6004 Telephone: (08) 9278 0300 Facsimile: (08) 9278 0585 Reference Details The recommended reference for this publication is: Water and Rivers Commission 1999, De Grey River Water Reserve Water Source Protection Plan, Port Hedland Regional Water Supply, Water and Rivers Commission, Water Resource Protection Series No WRP 24. -

NASA Satellite Sees Blake's Remnants Bringing Desert Rain to Western Australia 10 January 2020, by Rob Gutro
NASA satellite sees Blake's remnants bringing desert rain to Western Australia 10 January 2020, by Rob Gutro southeastern corner of the region. The Australian Government Bureau of Meteorology (ABM) in Western Australia issued several flood warnings at 10:47 a.m. WST on Friday Jan. 10. Flood Warnings were in effect for four areas. There is a Major Flood Warning for the De Grey River Catchment and a Flood Warning for the Fortescue River, Salt Lakes District Rivers, and southwestern parts the Sandy Desert Catchment. ABM said, "Major flooding is occurring in the Nullagine River in the De Grey river catchment. Most upstream locations have now peaked with minor to moderate flooding expected to continue during Friday before flooding starts to ease On Jan. 10, 2020, the MODIS instrument that flies throughout the area over the weekend. Heavy aboard NASA’s Aqua satellite provided a visible image rainfall from ex-Tropical Cyclone Blake has resulted of Ex-tropical storm Blake covering part of Western in in rapid river level rises, and areas of flooding Australia and still generating enough precipitation to call throughout the De Grey river catchment. Flooding for warnings. Credit: NASA Worldview has adversely impacted road conditions particularly at floodways resulting in multiple road closures." Rainfall totals over 24 hours in the De Grey NASA's Aqua satellite provided a look at the catchment indicated 1.30 inches (33 mm) at remnant clouds and storms associated with Ex- Nullagine. tropical Cyclone Blake as it continues to move through Western Australia and generate rainfall On Jan. 10, areas of flooding were occurring in the over desert areas. -

Systematic Taxonomy and Biogeography of Widespread
Systematics and Biogeography of Three Widespread Australian Freshwater Fish Species. by Bernadette Mary Bostock B.Sc. (Hons) Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy Deakin University February 2014 i ABSTRACT The variation within populations of three widespread and little studied Australian freshwater fish species was investigated using molecular genetic techniques. The three species that form the focus of this study are Leiopotherapon unicolor, Nematalosa erebi and Neosilurus hyrtlii, commonly recognised as the three most widespread Australian freshwater fish species, all are found in most of the major Australian drainage basins with habitats ranging from clear running water to near stagnant pools. This combination of a wide distribution and tolerance of a wide range of ecological conditions means that these species are ideally suited for use in investigating phylogenetic structure within and amongst Australian drainage basins. Furthermore, the combination of increasing aridity of the Australian continent and its diverse freshwater habitats is likely to promote population differentiation within freshwater species through the restriction of dispersal opportunities and localised adaptation. A combination of allozyme and mtDNA sequence data were employed to test the null hypothesis that Leiopotherapon unicolor represents a single widespread species. Conventional approaches to the delineation and identification of species and populations using allozyme data and a lineage-based approach using mitochondrial 16S rRNA sequences were employed. Apart from addressing the specific question of cryptic speciation versus high colonisation potential in widespread inland fishes, the unique status of L. unicolor as both Australia’s most widespread inland fish and most common desert fish also makes this a useful species to test the generality of current biogeographic hypotheses relating to the regionalisation of the Australian freshwater fish fauna. -

Birdwatching Around Geraldton and Dongara
1 CHAPMAN RIVER REGIONAL PARK Birdwatching around Geraldton and Dongara This extensive reserve within Geraldton City is worth Areas within the City of Geraldton provide eucalypt a visit at any time of year. It extends from Sunset & acacia woodland, river margins and shorelines. Birdwatching Beach north of Geraldton, along the Chapman River For the more adventurous, those with more time to to a large area of bushland and includes estuarine spare or en route to other places, there are a number salt-marsh, limestone cliff-tops, groves of river of other options, including significant river pools around Geraldton sheoak, eucalypts and acacia; scrub and heathlands. and estuaries, farmlands, ranges and bush reserves. Australian Pelican, Osprey, herons, egrets, White- The Houtman Abrolhos Islands to the west provide browed Scrubwren, Mistletoebird and Grey habitat and food sources for many seabirds. Currawong have all been recorded here. Please note: Directions are given for travel from Geraldton. Tappak Street P Sunset Beach Chapman River Local Contacts P BirdLife Midwest – Geraldton: P Jan Ph 9964 3773 Spalding Mike Ph 0427 872 161 Park Entrance P P Spalding Oval Crowtherton P Street Green Street P Acknowledgements: P Farlan Street Illustrations / photographs by Robin Ashford, John Anderson, Alan Collins, Pam Free, Keith Lightbody, Chapman River Regional Park Michael Morcombe, SusanTingay. Kempton Street Information: J. Checker, S. Vigilante, M. Lawrie - Chapman Road North West Costal Highway sites and bird lists; J. Brooker, A Howitt (Chapman Koojarra Street River Friends), N. Dunlop (Abrolhos). P Tersonia Way Webberton Road P Guide No 11AB Strathalbyn Road All content is subject to copyright ©. -

Coastal Sediment Cells for the Mid-West Coast
Department of Transport Coastal Sediment Cells for the Mid-West Coast Between the Moore River and Glenfield Beach, Western Australia Report Number: M 2014 07001 Version: 0 Date: September 2014 Recommended citation Stul T, Gozzard JR, Eliot IG and Eliot MJ (2014) Coastal Sediment Cells for the Mid-West Region between the Moore River and Glenfield Beach, Western Australia. Report prepared by Seashore Engineering Pty Ltd and Geological Survey of Western Australia for the Western Australian Department of Transport, Fremantle. The custodian of the digital dataset is the Department of Transport. Photographs used are from WACoast30. The Department of Transport acknowledges Bob Gozzard from Geological Survey of Western Australia for providing the images. Geological Survey of S e a s h o r e E n g i n e e r i n g Western Australia 2 Executive summary The aim of this report is to identify a hierarchy of sediment cells to assist planning, management, engineering, science and governance of the Mid-West coast. Sediment cells are spatially discrete areas of the coast within which marine and terrestrial landforms are likely to be connected through processes of sediment exchange, often described using sediment budgets. They include areas of sediment supply (sources), sediment loss (sinks), and the sediment transport processes linking them (pathways). Sediment transport pathways include both alongshore and cross-shore processes, and therefore cells are best represented in two-dimensions. They are natural management units with a physical basis and commonly cross jurisdictional boundaries. Sediment cells provide a summary of coastal data in a simple format and can be used to: 1. -

Flood Watch Areas
! Daly Sir Graham Pine Moore River Flood Watch Area No. Flood Watch Area No. ! Creek Island ! Ashburton River 12 Middle Onslow Coast 9 Osborne Flood Watch Areas Bigge Island Avon River 24 Pilbara Coastal Rivers 7 ! Kalumburu Island Ka!therine ! Western Australia Blackwood River 30 Preston River 29 Bamyili ! Busselton Coastal Rivers 31 Coronation Adolphus Salt Lakes District Rivers 20 Island Island Collie River 28 Augustus 1 Sandy Desert 10 Island De Grey River 8 ! Wyndham South Coastal Rivers 32 East Kimberley Rivers 2 ! South West District Rivers 33 Koolan Kununurra Fitzroy River 4 Island Sturt Creek District 5 Fortescue River 11 2 NT Swan River 25 Dunmarra Gascoyne River 15 3 ! ! Tanami Desert 6 Mount Greenough River 19 Barnett ! Warburton District Rivers 18 Warmun ! (Turkey Kalkarindji Harvey River 27 Derby ! Elliott West Kimberley Rivers 3 3 Creek) ! Lyndon-Minilya Rivers 13 Broome Western Desert 14 ! Looma Halls Moore and Hill Rivers 22 ! Fitzroy Renner 4 Creek Wooramel River 16 Bidyadanga Crossing ! ! Lajamanu Springs Murchison River 17 ! ! I n d i a n Community Murray River 26 Yarra Yarra Lakes District 21 (Lagrange) ! Larrawa ! O c e a n North Kimberley Rivers 1 Nullarbor District Rivers 23 ! Warrego 5 ! ! Sandfire Balgo Tennant Port Hill Creek ! Pardoo ! Hedland ! Barrow Island ! Karratha 6 Dampier ! ! ! Whim Creek Marble Roebourne Bar ! 7 Telfer ! Onslow 8 ! ! Pannawonica Exmouth ! 10 9 Munjina ! Gorge Cotton Yuendumu Nanutarra ! ! Creek ! ! Tom Coral ! ! Price 11 ! Lyndon Papunya Bay ! ! ! Newman ! Paraburdoo Alice 12 Springs