Coccidioides Immitis and Posadasii; a Review of Their Biology, Genomics, Pathogenesis, and Host Immunity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coccidioides Immitis
24/08/2017 FUNGAL AGENTS CAUSING INFECTION OF THE LUNG Microbiology Lectures of the Respiratory Diseases Prepared by: Rizalinda Sjahril Microbiology Department Faculty of Medicine Hasanuddin University 2016 OVERVIEW OF CLINICAL MYCOLOGY . Among 150.000 fungi species only 100-150 are human pathogens 25 spp most common pathogens . Majority are saprophyticLiving on dead or decayed organic matter . Transmission Person to person (rare) SPORE INHALATION OR ENTERS THE TISSUE FROM TRAUMA Animal to person (rare) – usually in dermatophytosis 1 24/08/2017 OVERVIEW OF CLINICAL MYCOLOGY . Human is usually resistant to infection, unless: Immunoscompromised (HIV, DM) Serious underlying disease Corticosteroid/antimetabolite treatment . Predisposing factors: Long term intravenous cannulation Complex surgical procedures Prolonged/excessive antibacterial therapy OVERVIEW OF CLINICAL MYCOLOGY . Several fungi can cause a variety of infections: clinical manifestation and severity varies. True pathogens -- have the ability to cause infection in otherwise healthy individuals 2 24/08/2017 Opportunistic/deep mycoses which affect the respiratory system are: Cryptococcosis Aspergillosis Zygomycosis True pathogens are: Blastomycosis Seldom severe Treatment not required unless extensive tissue Coccidioidomycosis destruction compromising respiratory status Histoplasmosis Or extrapulmonary fungal dissemination Paracoccidioidomycosis COMMON PATHOGENS OBTAINED FROM SPECIMENS OF PATIENTS WITH RESPIRATORY DISEASE Fungi Common site of Mode of Infectious Clinical -

Turning on Virulence: Mechanisms That Underpin the Morphologic Transition and Pathogenicity of Blastomyces
Virulence ISSN: 2150-5594 (Print) 2150-5608 (Online) Journal homepage: http://www.tandfonline.com/loi/kvir20 Turning on Virulence: Mechanisms that underpin the Morphologic Transition and Pathogenicity of Blastomyces Joseph A. McBride, Gregory M. Gauthier & Bruce S. Klein To cite this article: Joseph A. McBride, Gregory M. Gauthier & Bruce S. Klein (2018): Turning on Virulence: Mechanisms that underpin the Morphologic Transition and Pathogenicity of Blastomyces, Virulence, DOI: 10.1080/21505594.2018.1449506 To link to this article: https://doi.org/10.1080/21505594.2018.1449506 © 2018 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group© Joseph A. McBride, Gregory M. Gauthier and Bruce S. Klein Accepted author version posted online: 13 Mar 2018. Submit your article to this journal Article views: 15 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=kvir20 Publisher: Taylor & Francis Journal: Virulence DOI: https://doi.org/10.1080/21505594.2018.1449506 Turning on Virulence: Mechanisms that underpin the Morphologic Transition and Pathogenicity of Blastomyces Joseph A. McBride, MDa,b,d, Gregory M. Gauthier, MDa,d, and Bruce S. Klein, MDa,b,c a Division of Infectious Disease, Department of Medicine, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison, WI 53792, USA; b Division of Infectious Disease, Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, 1675 Highland Avenue, Madison, WI 53792, USA; c Department of Medical Microbiology and Immunology, University of Wisconsin School of Medicine and Public Health, 1550 Linden Drive, Madison, WI 53706, USA. -

25 Chrysosporium
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Universidade do Minho: RepositoriUM 25 Chrysosporium Dongyou Liu and R.R.M. Paterson contents 25.1 Introduction ..................................................................................................................................................................... 197 25.1.1 Classification and Morphology ............................................................................................................................ 197 25.1.2 Clinical Features .................................................................................................................................................. 198 25.1.3 Diagnosis ............................................................................................................................................................. 199 25.2 Methods ........................................................................................................................................................................... 199 25.2.1 Sample Preparation .............................................................................................................................................. 199 25.2.2 Detection Procedures ........................................................................................................................................... 199 25.3 Conclusion .......................................................................................................................................................................200 -

Valley Fever a K a Coccidioidomycosis Coccidioidosis Coccidiodal Granuloma San Joaquin Valley Fever Desert Rheumatism Valley Bumps Cocci Cox C
2019 Lung Infection Symposium - Libke 10/26/2019 58 YO ♂ • 1974 PRESENTED WITH HEADACHE – DX = COCCI MENINGITIS WITH HYDROCEPHALUS – Rx = IV AMPHOTERICIN X 6 WKS – VP SHUNT – INTRACISTERNAL AMPHO B X 2.5 YRS (>200 PUNCTURES) • 1978 – 2011 VP SHUNT REVISIONS X 5 • 1974 – 2019 GAINFULLY EMPLOYED, RAISED FAMILY, RETIRED AND CALLS OCCASIONALLY TO SEE HOW I’M DOING. VALLEY FEVER A K A COCCIDIOIDOMYCOSIS COCCIDIOIDOSIS COCCIDIODAL GRANULOMA SAN JOAQUIN VALLEY FEVER DESERT RHEUMATISM VALLEY BUMPS COCCI COX C 1 2019 Lung Infection Symposium - Libke 10/26/2019 COCCIDIOIDOMYCOSIS • DISEASE FIRST DESCRIBED IN 1892 – POSADAS –ARGENTINA – RIXFORD & GILCHRIST - CALIFORNIA – INITIALLY THOUGHT PARASITE – RESEMBLED COCCIDIA “COCCIDIOIDES” – “IMMITIS” = NOT MINOR COCCIDIOIDOMYCOSIS • 1900 ORGANISM IDENTIFIED AS FUNGUS – OPHULS AND MOFFITT – ORGANISM CULTURED FROM TISSUES OF PATIENT – LIFE CYCLE DEFINED – FULFULLED KOCH’S POSTULATES 2 2019 Lung Infection Symposium - Libke 10/26/2019 COCCIDIOIDOMYCOSIS • 1932 ORGANISM IN SOIL SAMPLE FROM DELANO – UNDER BUNKHOUSE OF 4 PATIENTS – DISEASE FATAL • 1937 DICKSON & GIFFORD CONNECTED “VALLEY FEVER” TO C. IMMITIS – USUALLY SELF LIMITED – FREQUENTLY SEEN IN SAN JOAQUIN VALLEY – RESPIRATORY TRACT THE PORTAL OF ENTRY The usual cause for coccidioidomycosis in Arizona is C. immitis A. True B. False 3 2019 Lung Infection Symposium - Libke 10/26/2019 COCCIDIOIDAL SPECIES • COCCIDIOIDES IMMITIS – CALIFORNIA • COCCIDIOIDES POSADASII – NON-CALIFORNIA • ARIZONA, MEXICO • OVERLAP IN SAN DIEGO AREA THE MICROBIAL WORLD • PRIONS -

Coccidioides Posadasii Contains Single Chitin Synthase Genes Corresponding to Classes I to VII
Fungal Genetics and Biology 43 (2006) 775–788 www.elsevier.com/locate/yfgbi Coccidioides posadasii contains single chitin synthase genes corresponding to classes I to VII M. Alejandra Mandel a,b, John N. Galgiani b,c,d, Scott Kroken a, Marc J. Orbach a,b,* a Department of Plant Sciences, Division of Plant Pathology and Microbiology, University of Arizona, Tucson, AZ 85721-0036, USA b Valley Fever Center for Excellence, Tucson, AZ, USA c Department of Internal Medicine, College of Medicine, University of Arizona, Tucson, AZ, USA d Southern Arizona Veterans Administration Health Care System, Tucson, AZ, USA Received 21 September 2005; accepted 23 May 2006 Available online 20 July 2006 Abstract Coccidioides posadasii is a dimorphic fungal pathogen of humans and other mammals. The switch between saprobic and parasitic growth involves synthesis of new cell walls of which chitin is a significant component. To determine whether particular subsets of chitin synthases (CHSes) are responsible for production of chitin at different stages of differentiation, we have isolated six CHS genes from this fungus. They correspond, together with another reported CHS gene, to single members of the seven defined classes of chitin synthases (classes I–VII). Using Real-Time RT-PCR we show their pattern of expression during morphogenesis. CpCHS2, CpCHS3, and CpCHS6 are preferentially expressed during the saprobic phase, while CpCHS1 and CpCHS4 are more highly expressed during the parasitic phase. CpCHS5 and CpCHS7 expression is similar in both saprobic and parasitic phases. Because C. posadasii contains single members of the seven classes of CHSes found in fungi, it is a good model to investigate the putatively different roles of these genes in fungal growth and differentiation. -

Valley Fever (Coccidioidomycosis) Tutorial for Primary Care Professionals, Now in Its Second Printing
Valley Fever (Coccidioidomycosis) Tutorial for Primary Care Professionals Prepared by the VALLEY FEVER CENTER FOR EXCELLENCE The University of Arizona 2 TABLE OF CONTENTS Preface ....................................................................................... 4 SECTION 1 ................................................................................. 7 OVERVIEW OF COCCIDIOIDOMYCOSIS History .................................................................................... 8 Mycology ................................................................................ 8 Epidemiology ....................................................................... 10 Spectrum of disease ........................................................... 11 Current therapies ................................................................. 12 SECTION 2 ............................................................................... 13 THE IMPORTANCE OF VALLEY FEVER IN PRIMARY CARE Case reporting ..................................................................... 14 Value of early diagnosis ....................................................... 17 SECTION 3 ............................................................................... 19 PRIMARY CARE MANAGEMENT OF COCCIDIOIDOMYCOSIS C onsider the diagnosis ........................................................ 20 O rder the right tests ............................................................. 23 C heck for risk factors .......................................................... 29 C heck for complications -

25 Chrysosporium
25 Chrysosporium Dongyou Liu and R.R.M. Paterson contents 25.1 Introduction ..................................................................................................................................................................... 197 25.1.1 Classification and Morphology ............................................................................................................................ 197 25.1.2 Clinical Features .................................................................................................................................................. 198 25.1.3 Diagnosis ............................................................................................................................................................. 199 25.2 Methods ........................................................................................................................................................................... 199 25.2.1 Sample Preparation .............................................................................................................................................. 199 25.2.2 Detection Procedures ........................................................................................................................................... 199 25.3 Conclusion .......................................................................................................................................................................200 References .................................................................................................................................................................................200 -

Fungal Serology Update – Test Algorithms and Methodologies
LABSTRACT – October 2019 Fungal Serology Update – Test algorithms and methodologies Audience Health care providers and laboratories involved in submission of specimens for fungal serology testing. Overview Beginning October 2019, the fungal serology testing algorithms and methodologies for Aspergillus, Histoplasma capsulatum, Blastomyces dermatitidis and Coccidiodies immitis used at Public Health Ontario have changed. All assays are now Health Canada licensed. Complement fixation (CF) assays have been discontinued. Summary of fungal serology assay methods now in use at PHO Laboratory Serologic Test Method used at PHO Analyte Detected Ordered Aspergillus Enzyme Immunoassay (EIA) IgG antibodies Histoplasma Immunodiffusion M and H band precipitins Blastomyces Immunodiffusion A band precipitins IgM (against TP antigens) and Screen, EIA IgG (against CF antigens) Coccidioides IDTP and IDCF band Confirmation, Immunodiffusion precipitins Page 1 of 4 Fungal Serology Update – Test algorithms and methodologies LAB-SD-134-000 Background information Please note, culture remains the gold standard for the diagnosis of most invasive fungal infections. However, fungal infections can be challenging to diagnose as symptoms are often non-specific and can mimic bacterial, viral, other fungal infections or malignancy. Results of fungal serological tests can be used to aid in the diagnosis and/or management of specific fungal infections and fungal allergies. Aspergillus species are ubiquitous environmental moulds which people inhale each day. While invasive disease is typically restricted to immunocompromised individuals, immunocompetent individuals can have certain allergic manifestations, or develop chronic pulmonary aspergillosis (CPA). Histoplasma capsulatum, Blastomyces dermatitidis and Coccidioides immitis are endemic, dimorphic fungi which can infect otherwise healthy individuals. Most infected individuals are exposed by inhaling fungal spores from the environment and present with initial clinical symptoms of respiratory illness. -

Penicillium Marneffei
DIFFERENTIAL EXPRESSION OF GENES ENCODING SECRETED PROTEINS IN PENICILLIUM MARNEFFEI by Suzie Rezenom Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Biological Sciences Program YOUNGSTOWN STATE UNIVERSITY May, 2013 DIFFERENTIAL EXPRESSION OF GENES ENCODING SECRETED PROTEINS IN PENICILLIUM MARNEFFEI Suzie Rezenom I hereby release this thesis to the public. I understand that this thesis will be made available from the OhioLINK ETD Center and the Maag Library Circulation Desk for public access. I also authorize the University or other individuals to make copies of this thesis as needed for scholarly research. Signature: ______________________________________________________________________ Suzie Rezenom, Student Date Approvals: _______________________________________________________________________ Dr. Chester R Cooper, Jr. Thesis Advisor Date _______________________________________________________________________ Dr. Xiangjia Min, Committee Member Date _______________________________________________________________________ Dr. Jonathan Caguiat, Committee Member Date _______________________________________________________________________ Dr. Brian Depoy, Dean of School of Graduate Studies & Research Date ABSTRACT Penicillium marneffei is a dimorphic pathogenic fungus endemic to Southeast Asia. It primarly infects individuals with compromised immunity, particularly those with AIDS. This fungus undergoes a dimorphic switch when shifted from growth at 25ºC to 37ºC. At 25ºC, P. marneffei grows -

Fungal Infections in PIDD Patients
Funga l Infections in PIDD Patients PIDD patients are more prone to certain types of infections. Knowing which ones they are most susceptible to and how they are most commonly treated can help to minimize the risk. By Alexandra F. Freeman, MD, and Anahita Agharahimi, MSN, CRNP 16 December-January 2014 www.IGLiving.com IG Living! ndividuals with primary immune deficiencies (PID Ds) are (paronychia) or the finger nails and toenails themselves at greater risk for recurrent infections compared with (onychomycosis). Candida can enter the bloodstream and Ithose with normal immune systems. PIDD patients cause more severe infections when the normal skin barriers fre quently have a genetic defect that causes an abnormality are compromised such as with central venous access lines in the number and/or function of one or more compo - (long-term IV access) that are sometimes needed for various nents of the immune system that fights infections. These treatments. These invasive infections usually cause infections can be predominantly viral, bacterial or fungal, fever and more acute illness compared with the more depending on the type of white blood cells affected by the mild infections such as thrush and vaginal yeast infections. specific immune deficiency . For instance, neutrophil Ringworm is also caused by yeasts, including Trichophyton abnormalities lead to recurrent bacterial and mold infections ; and Microsporum species, that cause rashes on the skin or B lymphocytes typically lead to bacterial infections, more scalp. Tinea versicolor is caused by the yeast Malassezia specifically those that antibodies prevent such as furfur and causes a rash usually on the trunk. -
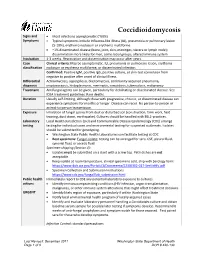
Coccidioidomycosis Reporting and Investigation Guideline
Coccidioidomycosis Signs and • Most infections asymptomatic (~60%) Symptoms • Typical symptoms include influenza-like illness (ILI), pneumonia or pulmonary lesion (5-10%), erythema nodosum or erythema multiforme • ~1% disseminated disease (bone, joint, skin, meninges, viscera or lymph node); dissemination more likely for men, some racial groups, altered immune system Incubation 1-3 weeks. Reactivation and dissemination may occur after years. Case Clinical criteria: May be asymptomatic. ILI, pneumonia or pulmonary lesion, erythema classification nodosum or erythema multiforme, or disseminated infection. Confirmed: Positive IgM, positive IgG, positive culture, or skin-test conversion from negative to positive after onset of clinical illness Differential Actinomycosis, aspergillosis, blastomycosis, community acquired pneumonia, diagnosis cryptococcosis, histoplasmosis, meningitis, sarcoidosis, tuberculosis, malignancy Treatment Antifungal agents can be given, particularly for debilitating or disseminated disease. See IDSA treatment guidelines. Rare deaths. Duration Usually self-limiting, although those with progressive, chronic, or disseminated disease can experience symptoms for months or longer. Disease can recur. No person-to-person or animal-to-person transmission. Exposure Inhalation of fungal spores from dust or disturbed soil (construction, farm work, field training, dust storm, earthquake). Cultures should be handled with BSL2-practices. Laboratory Local Health Jurisdiction (LHJ) and Communicable Disease Epidemiology (CDE) arrange testing testing for individual cases and environmental testing for suspected outbreaks. Isolates should be submitted for genotyping. • Washington State Public Health Laboratories can facilitate testing at CDC • Best specimens: Fungal isolate; testing can be arranged for sera, CSF, pleural fluid, synovial fluid, or ascetic fluid Specimen shipping (Section 4): • Isolates must be submitted on a slant with a screw top. Petri dishes are not acceptable. -

Disseminated Infection Due to Chrysosporium Zonatum in A
JOURNAL OF CLINICAL MICROBIOLOGY, Jan. 1999, p. 18–25 Vol. 37, No. 1 0095-1137/99/$04.0010 Copyright © 1999, American Society for Microbiology. All Rights Reserved. Disseminated Infection Due to Chrysosporium zonatum in a Patient with Chronic Granulomatous Disease and Review of Non-Aspergillus Fungal Infections in Patients with This Disease EMMANUEL ROILIDES,1* LYNNE SIGLER,2 EVANGELIA BIBASHI,3 HELEN KATSIFA,1 4 1 NICOLAS FLARIS, AND CHRISTOS PANTELIADIS Third Department of Pediatrics, Aristotle University of Thessaloniki,1 and Microbiology3 and Pathology Departments,4 Hippokration Hospital, Thessaloniki, Greece, and Microfungus Collection and Herbarium, Devonian Botanic Garden, University of Alberta, Edmonton, Alberta, Canada2 Received 15 June 1998/Returned for modification 1 August 1998/Accepted 6 October 1998 We report the first case of Chrysosporium zonatum infection in a 15-year-old male with chronic granuloma- tous disease who developed a lobar pneumonia and tibia osteomyelitis while on prophylaxis with gamma inter- feron. The fungus was isolated from sputum and affected bone, and hyphae were observed in the bone by his- topathology. Therapy with amphotericin B eradicated the osteomyelitis and pneumonia, but pneumonia recurred in association with pericarditis and pleuritis during therapy with itraconazole. These manifestations subsided, and no recurrences occurred with liposomal amphotericin B therapy. Infections caused by Chrysosporium spe- cies are very rare, and C. zonatum has not previously been reported to cause mycosis in humans. This species, the anamorph of the heterothallic ascomycete Uncinocarpus orissi (family Onygenaceae), is distinguished by its thermotolerance, by colonies which darken from yellowish white to buff, and by club-shaped terminal aleurio- conidia borne at the ends of short, typically curved stalks.