E-Binder March 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endothelin System and Therapeutic Application of Endothelin Receptor
xperim ACCESS Freely available online & E en OPEN l ta a l ic P in h l a C r m f o a c l a o n l o r g u y o J Journal of ISSN: 2161-1459 Clinical & Experimental Pharmacology Research Article Endothelin System and Therapeutic Application of Endothelin Receptor Antagonists Abebe Basazn Mekuria, Zemene Demelash Kifle*, Mohammedbrhan Abdelwuhab Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia ABSTRACT Endothelin is a 21 amino acid molecule endogenous potent vasoconstrictor peptide. Endothelin is synthesized in vascular endothelial and smooth muscle cells, as well as in neural, renal, pulmonic, and inflammatory cells. It acts through a seven transmembrane endothelin receptor A (ETA) and endothelin receptor B (ETB) receptors belongs to G protein-coupled rhodopsin-type receptor superfamily. This peptide involved in pathogenesis of cardiovascular disorder like (heart failure, arterial hypertension, myocardial infraction and atherosclerosis), renal failure, pulmonary arterial hypertension and it also involved in pathogenesis of cancer. Potentially endothelin receptor antagonist helps the treatment of the above disorder. Currently, there are a lot of trails both per-clinical and clinical on endothelin antagonist for various cardiovascular, pulmonary and cancer disorder. Some are approved by FAD for the treatment. These agents are including both selective and non-selective endothelin receptor antagonist (ETA/B). Currently, Bosentan, Ambrisentan, and Macitentan approved -

Endothelin-Receptor Antagonists Are Proapoptotic and Antiproliferative in Human Colon Cancer Cells
British Journal of Cancer (2003) 88, 788 – 795 & 2003 Cancer Research UK All rights reserved 0007 – 0920/03 $25.00 www.bjcancer.com Endothelin-receptor antagonists are proapoptotic and antiproliferative in human colon cancer cells 1 1 ,1 L Peduto Eberl , R Bovey and L Juillerat-Jeanneret* 1 University Institute of Pathology, CHUV, University of Lausanne, Bugnon 25, CH1011 Lausanne, Switzerland Endothelin (ET)-1 can act as an autocrine/paracrine growth factor or an antiapoptotic factor in human cancers. To study the role of ET-1 in human colon cancer, proliferation and apoptosis of colon carcinoma cells was investigated using human HT-29 and SW480 colon carcinoma cells. ET-1 was secreted by these cells. Treatment of cells with bosentan, a dual ETA/B-receptor antagonist, decreased cell number. Inhibition of DNA synthesis by bosentan was observed only in the presence of serum. Exogenously added ET-1 did not increase DNA synthesis in serum-deprived cells. SW480 cells were sensitive and HT-29 cells were resistant to FasL- induced apoptosis. Bosentan sensitised resistant HT-29 cells to FasL-induced, caspase-mediated apoptosis, but not to TNF-a-induced apoptosis. Bosentan and/or FasLigand (FasL) did not modulate the expression of caspase-8 or FLIP. Bosentan sensitisation to À13 À10 À9 À7 apoptosis was reversed by low concentrations (10 –10 M), but not by high concentrations (10 –10 M) of ET-1. These results suggest that the binding of ET-1 to high-affinity sites inhibits FasL-induced apoptosis, while the binding of either ET-1 or receptor antagonists to low-affinity sites promotes FasL-induced apoptosis. -

Self-Nano-Emulsifying Drug-Delivery Systems: from the Development to the Current Applications and Challenges in Oral Drug Delivery
pharmaceutics Review Self-Nano-Emulsifying Drug-Delivery Systems: From the Development to the Current Applications and Challenges in Oral Drug Delivery Aristote B. Buya 1,2 , Ana Beloqui 1, Patrick B. Memvanga 2 and Véronique Préat 1,* 1 Advanced Drug Delivery and Biomaterials, Louvain Drug Research Institute, Université Catholique de Louvain, Avenue Mounier 73, B1.73.12, 1200 Brussels, Belgium; [email protected] (A.B.B.); [email protected] (A.B.) 2 Pharmaceutics and Phytopharmaceutical Drug Development Research Group, Faculty of Pharmaceutical Sciences, University of Kinshasa, Kinshasa XI BP 212, Democratic Republic of the Congo; [email protected] * Correspondence: [email protected]; Tel.: +32-27647309 Received: 3 November 2020; Accepted: 5 December 2020; Published: 9 December 2020 Abstract: Approximately one third of newly discovered drug molecules show insufficient water solubility and therefore low oral bio-availability. Self-nano-emulsifying drug-delivery systems (SNEDDSs) are one of the emerging strategies developed to tackle the issues associated with their oral delivery. SNEDDSs are composed of an oil phase, surfactant, and cosurfactant or cosolvent. SNEDDSs characteristics, their ability to dissolve a drug, and in vivo considerations are determinant factors in the choice of SNEDDSs excipients. A SNEDDS formulation can be optimized through phase diagram approach or statistical design of experiments. The characterization of SNEDDSs includes multiple orthogonal methods required to fully control SNEDDS manufacture, stability, and biological fate. Encapsulating a drug in SNEDDSs can lead to increased solubilization, stability in the gastro-intestinal tract, and absorption, resulting in enhanced bio-availability. The transformation of liquid SNEDDSs into solid dosage forms has been shown to increase the stability and patient compliance. -

Comparison of Pharmacological Activity of Macitentan and Bosentan in Preclinical Models of Systemic and Pulmonary Hypertension
LFS-13929; No of Pages 7 Life Sciences xxx (2014) xxx–xxx Contents lists available at ScienceDirect Life Sciences journal homepage: www.elsevier.com/locate/lifescie Comparison of pharmacological activity of macitentan and bosentan in preclinical models of systemic and pulmonary hypertension Marc Iglarz ⁎, Alexandre Bossu, Daniel Wanner, Céline Bortolamiol, Markus Rey, Patrick Hess, Martine Clozel Drug Discovery Department, Actelion Pharmaceuticals Ltd, Gewerbestrasse 16, 4123 Allschwil, Switzerland article info abstract Article history: Aims: The endothelin (ET) system is a tissular system, as the production of ET isoforms is mostly autocrine or Received 29 October 2013 paracrine. Macitentan is a novel dual ETA/ETB receptor antagonist with enhanced tissue distribution and Accepted 12 February 2014 sustained receptor binding properties designed to achieve a more efficacious ET receptor blockade. To determine Available online xxxx if these features translate into improved efficacy in vivo, a study was designed in which rats with either systemic or pulmonary hypertension and equipped with telemetry were given macitentan on top of maximally effective Keywords: doses of another dual ET /ET receptor antagonist, bosentan, which does not display sustained receptor occupan- Endothelin A B Pharmacology cy and shows less tissue distribution. – Blood pressure Main methods: After establishing dose response curves of both compounds in conscious, hypertensive Dahl salt- Pulmonary hypertension sensitive and pulmonary hypertensive bleomycin-treated rats, macitentan was administered on top of the max- Rat imal effective dose of bosentan. Key findings: In hypertensive rats, macitentan 30 mg/kg further decreased mean arterial blood pressure (MAP) by 19 mm Hg when given on top of bosentan 100 mg/kg (n =9,p b 0.01 vs. -

Summary of Appeals & Independent Review Organization
All Other Appeals All other appeals are for drugs not in an inpatient hospital setting that Molina was not able to approve. Sometimes, the clinical information sent to us for these drugs do not meet medical necessity on initial review. When drug preauthorization requests are denied, a member or provider has the right to appeal. Appeals allow time to provide more clinical information. With complete clinical information, we can usually approve the drug. These are considered an appeal overturn. When the denial decision is not overturned, it is considered upheld. Service Code/Drug Name Service Code Description Number of Appeals Number of Appeals Total Appeals Upheld Overturned A9274 EXTERNAL AMB INSULIN DEL SYSTEM DISPOSABLE EA 0 1 1 Abatacept 3 1 4 Abemaciclib 1 0 1 Acalabrutinib 0 1 1 Acne Combination - Two Ingredient 1 0 1 Acyclovir 0 1 1 Adalimumab 7 14 21 Adrenergic Combination - Two Ingredient 1 0 1 Aflibercept 0 2 2 Agalsidase 1 0 1 Alfuzosin 1 0 1 Amantadine 1 0 1 Ambrisentan 0 1 1 Amphetamine 0 1 1 Amphetamine Mixtures - Two Ingredient 1 8 9 Apixaban 7 21 28 Apremilast 12 13 25 Aprepitant 0 1 1 Aripiprazole 5 9 14 ARNI-Angiotensin II Recept Antag Comb - Two Ingredient 6 9 15 Asenapine 0 1 1 Atomoxetine 1 3 4 Atorvastatin 0 1 1 Atovaquone 1 0 1 Axitinib 0 1 1 Azathioprine 0 1 1 Azilsartan 1 0 1 Azithromycin 1 0 1 Baclofen 0 1 1 Baricitinib 1 1 2 Belimumab 0 1 1 Benralizumab 1 0 1 Beta-blockers - Ophthalmic Combination - Two Ingredient 0 2 2 Bimatoprost 0 1 1 Botulinum Toxin 1 4 5 Buprenorphine 4 3 7 Calcifediol 1 0 1 Calcipotriene -
Download Leaflet View the Patient Leaflet in PDF Format
long as you are taking Bosentan. We suggest you write the date of your most recent test and also of your next test (ask your doctor for the date) on the Patient Alert Package leaflet: Information for the user Card, to help you remember when your next test is due. Bosentan 62.5 mg film-coated tablets Blood tests for liver function These will be done every month for the duration of Bosentan 125 mg film-coated tablets treatment with Bosentan. After an increase in dose an additional test will be done after 2 weeks. bosentan Blood tests for anaemia Read all of this leaflet carefully before you start These will be done every month for the first 4 months taking this medicine because it contains important of treatment, then every 3 months after that, as patients information for you. taking Bosentan may get anaemia. - Keep this leaflet. You may need to read it again. If these results are abnormal, your doctor may decide - If you have any further questions, ask your doctor to reduce your dose or stop treatment with Bosentan or pharmacist. and to perform further tests to investigate the cause. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if Children and adolescents their signs of illness are the same as yours. Bosentan is not recommended in paediatric patients - If you get any side effects, talk to your doctor or with systemic sclerosis and ongoing digital ulcer pharmacist. This includes any possible side effects disease. Please see also section 3. -
![TRACLEER® [Bosentan] 62.5 Mg and 125 Mg Film-Coated Tablets](https://docslib.b-cdn.net/cover/9612/tracleer%C2%AE-bosentan-62-5-mg-and-125-mg-film-coated-tablets-1209612.webp)
TRACLEER® [Bosentan] 62.5 Mg and 125 Mg Film-Coated Tablets
TRACLEER® [bosentan] 62.5 mg and 125 mg film-coated tablets Use of TRACLEER® requires attention to two significant concerns: 1) potential for serious liver injury, and 2) potential damage to a fetus. WARNING: Potential liver injury TRACLEER® causes at least 3-fold (upper limit of normal; ULN) elevation of liver aminotransferases (ALT and AST) in about 11% of patients, accompanied by elevated bilirubin in a small number of cases. Because these changes are a marker for potential serious liver injury, serum aminotransferase levels must be measured prior to initiation of treatment and then monthly (see WARNINGS: Potential Liver Injury and DOSAGE AND ADMINISTRATION). To date, in a setting of close monitoring, elevations have been reversible, within a few days to 9 weeks, either spontaneously or after dose reduction or discontinuation, and without sequelae. Elevations in aminotransferases require close attention (see DOSAGE AND ADMINISTRATION). TRACLEER® should generally be avoided in patients with elevated aminotransferases (> 3 x ULN) at baseline because monitoring liver injury may be more difficult. If liver aminotransferase elevations are accompanied by clinical symptoms of liver injury (such as nausea, vomiting, fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or increases in bilirubin ≥ 2 x ULN, treatment should be stopped. There is no experience with the re-introduction of TRACLEER® in these circumstances. CONTRAINDICATION: Pregnancy TRACLEER® (bosentan) is very likely to produce major birth defects if used by pregnant women, as this effect has been seen consistently when it is administered to animals (see CONTRAINDICATIONS). Therefore, pregnancy must be excluded before the start of treatment with TRACLEER® and prevented thereafter by the use of a reliable method of contraception. -

Ambrisentan (Letairis®) Issued by PHA’S Scientific Leadership Council Information Is Based on the United States Food and Drug Administration Drug Labeling
Ambrisentan (Letairis®) Issued by PHA’s Scientific Leadership Council Information is based on the United States Food and Drug Administration drug labeling Last Updated November 2013 WHAT IS AMBRISENTAN? Ambrisentan is an oral medication classified as an endothelin receptor antagonist (ERA) which is approved for the treatment of pulmonary arterial hypertension (PAH) in World Health Organization (WHO) Group 1 patients. The goal of this therapy is to improve exercise ability and slow progression of the disease. Ambrisentan was approved for PAH by the United States Food and Drug Administration (FDA) in 2007. HOW DOES AMBRISENTAN WORK? Ambrisentan works by blocking endothelin, a substance made by the body. Endothelin causes blood vessels to narrow (constrict). It also causes abnormal growth of the muscle in the walls of the blood vessels in the lungs. This narrowing increases the pressure required to push the blood through the lungs to get oxygen. By blocking the action of endothelin, causing vessels to relax, ambrisentan decreases the pulmonary blood pressure to the heart and improves its function. This generally results in the ability to be more active. Research studies have verified this improvement. HOW IS AMBRISENTAN GIVEN? Ambrisentan is taken orally, with or without food. There are two FDA approved doses; 5 mg (pale pink and square) or 10 mg (dark pink and oval). Patients’ physicians will decide which strength is right for them. HOW IS AMBRISENTAN SUPPLIED? Ambrisentan comes in 5 and 10 mg, film-coated, unscored tablets. The 5 mg tablet is pale pink and square. The 10 mg tablet is deep pink and oval. -

Ambrisentan (Letairis) Reference Number: ERX.SPMN.84 Effective Date: 07/16 Last Review Date: 06/16 Revision Log
Clinical Policy: Ambrisentan (Letairis) Reference Number: ERX.SPMN.84 Effective Date: 07/16 Last Review Date: 06/16 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Policy/Criteria It is the policy of health plans affiliated with Envolve Pharmacy Solutions® that ambrisentan (Letairis®) is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Pulmonary Arterial Hypertension (PAH) (must meet all): 1. Prescribed by or in consultation with a cardiologist or pulmonologist experienced in the diagnosis and treatment of pulmonary hypertension; 2. Diagnosis of PAH World Health Organization (WHO) Group 1 (appendix B) confirmed by right heart catheterization and with one of the following: a. Inadequate response or contraindication to acute vasodilator testing; b. Trial and failure of, or contraindication to, calcium channel blockers; 3. WHO/New York Heart Association (NYHA) functional class II, III, or IV (appendix C); 4. Prescribed dose of Letairis does not exceed 10 mg once daily. Approval Duration: 3 months B. Other diagnoses/indications: Refer to ERX.SPMN.16 - Global Biopharm Policy. II. Continued Approval A. Pulmonary Arterial Hypertension (PAH) (must meet all): 1. Currently receiving medication via health plan benefit or member has previously met all initial approval criteria. Approval Duration: 6 months B. Other diagnoses/indications (must meet 1 or 2): 1. Currently receiving medication via health plan benefit and documentation supports positive response to therapy; or 2. Refer to ERX.SPMN.16 - Global Biopharm Policy. Page 1 of 5 CLINICAL POLICY Ambrisentan Background Description/Mechanism of Action: Letairis is the brand name for ambrisentan, an endothelin receptor antagonist that is selective for the endothelin type-A (ETA) receptor. -
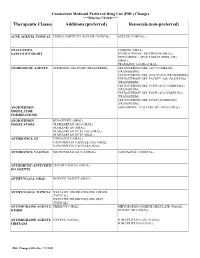
Therapeutic Classes Additions (Preferred) Removals (Non-Preferred)
Connecticut Medicaid Preferred Drug List (PDL) Changes ***Effective 7/1/2021*** Therapeutic Classes Additions (preferred) Removals (non-preferred) ACNE AGENTS, TOPICAL EPIDUO FORTE GEL W/PUMP (TOPICAL) AZELEX (TOPICAL) ANALGESICS, CODEINE (ORAL) NARCOTICS SHORT HYDROCODONE / IBUPROFEN (ORAL) OXYCODONE / APAP TABLET (PROLATE) (ORAL) TRAMADOL 100 MG (ORAL) ANDROGENIC AGENTS ANDROGEL GEL PUMP (TRANSDERM) TESTOSTERONE GEL (AG) (VOGELXO) (TRANSDERM) TESTOSTERONE GEL (VOGELXO) (TRANSDERM) TESTOSTERONE GEL PACKET (AG) (VOGELXO) (TRANSDERM) TESTOSTERONE GEL PUMP (AG) (ANDROGEL) (TRANSDERM) TESTOSTERONE GEL PUMP (AG) (VOGELXO) (TRANSDERM) TESTOSTERONE GEL PUMP (ANDROGEL) (TRANSDERM) ANGIOTENSIN AMLODIPINE / VALSARTAN / HCTZ (ORAL) MODULATOR COMBINATIONS ANGIOTENSIN BENAZEPRIL (ORAL) MODULATORS OLMESARTAN (AG) (ORAL) OLMESARTAN (ORAL) OLMESARTAN HCTZ (AG) (ORAL) OLMESARTAN HCTZ (ORAL) ANTIBIOTICS, GI TINIDAZOLE (ORAL) VANCOMYCIN CAPSULE (AG) (ORAL) VANCOMYCIN CAPSULE (ORAL) ANTIBIOTICS, VAGINAL METRONIDAZOLE (VAGINAL) VANDAZOLE (VAGINAL) ANTIEMETIC/ANTIVERTI EMEND CAPSULE (ORAL) GO AGENTS ANTIFUNGALS, ORAL NOXAFIL TABLET (ORAL) ANTIFUNGALS, TOPICAL NYSTATIN-TRIAMCINOLONE CREAM (TOPICAL) NYSTATIN-TRIAMCINOLONE OINT (TOPICAL) ANTIMIGRAINE AGENTS, UBRELVY (ORAL) DIHYDROERGOTAMINE MESYLATE (NASAL) OTHER NURTEC ODT (ORAL) ANTIMIGRAINE AGENTS, IMITREX (NASAL) SUMATRIPTAN (AG) (NASAL) TRIPTANS SUMATRIPTAN (NASAL) PDL Changes Effective: 7/1/2021 Connecticut Medicaid Preferred Drug List (PDL) Changes ***Effective 7/1/2021*** Therapeutic Classes Additions -

84 November 2008 Produced by NHS Tayside Drug and Therapeutics Committee Medicines Advisory Group (MAG)
TAYSIDE PRESCRIBER Tayside DTC Supplement No 84 November 2008 Produced by NHS Tayside Drug and Therapeutics Committee Medicines Advisory Group (MAG) SMC Advice issued in November 2008 Medicine Indication Local recommendation Comments and useful category links Ambrisentan tablets Treatment of patients with class Restricted for use within SMC advice (Volibris®) II and III pulmonary arterial national treatment centre SPC link hypertension to improve exercise capacity Anidulafungin powder and Treatment of invasive Pending AMG decision SMC advice solvent (Ecalta®) - candidiasis in adult non- SPC link Resubmission neutropenic patients Bosentan film-coated tablets Class II pulmonary arterial Not recommended SMC advice (Tracleer®) - hypertension Bosentan was previously Non submission accepted in 2003 for restricted use in grade III pulmonary arterial hypertension Icatibant solution for Symptomatic treatment of acute Not recommended SMC advice subcutaneous injection attacks of hereditary (Firazyr®) angioedema in adults Insulin glulisine solution for Treatment of adolescents and Non-formulary SMC advice subcutaneous injection children, 6 years or older with SPC link (Apidra®) - Abbreviated diabetes mellitus where a short Diabetes Handbook submission acting insulin analogue is appropriate Insulin lispro solution for Adults and children with Non-formulary SMC advice injection (Humalog® diabetes mellitus SPC link KwikPen) - Diabetes Handbook Abbreviated submission Insulin lispro (Humalog® Patients with diabetes mellitus, Non-formulary SMC advice Mix25 -

"Tracleer, INN- Bosentan"
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Tracleer 62.5 mg film-coated tablets Tracleer 125 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tracleer 62.5 mg film-coated tablets Each film-coated tablet contains 62.5 mg bosentan (as monohydrate). Tracleer 125 mg film-coated tablets Each film-coated tablet contains 125 mg bosentan (as monohydrate). Excipient with known effect This medicine contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablet (tablets): Tracleer 62.5 mg film-coated tablets Orange-white, round, biconvex, film-coated tablets, embossed with “62,5” on one side. Tracleer 125 mg film-coated tablets Orange-white, oval, biconvex, film-coated tablets, embossed with “125” on one side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Treatment of pulmonary arterial hypertension (PAH) to improve exercise capacity and symptoms in patients with WHO functional class III. Efficacy has been shown in: Primary (idiopathic and heritable) pulmonary arterial hypertension Pulmonary arterial hypertension secondary to scleroderma without significant interstitial pulmonary disease Pulmonary arterial hypertension associated with congenital systemic-to-pulmonary shunts and Eisenmenger’s physiology Some improvements have also been shown in patients with pulmonary arterial hypertension WHO functional class II (see section 5.1). Tracleer is also indicated to reduce the number of new digital ulcers in patients with systemic sclerosis and ongoing digital ulcer disease (see section 5.1). 2 4.2 Posology and method of administration Method of administration Tablets are to be taken orally morning and evening, with or without food.