Matrix Effects the Final Method Included 99 Synthetic Qcmed QC: Quality Control START / /STOP STOP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Swedish Code of Statutes
1. ------IND- 2018 0506 S-- EN- ------ 20190508 --- --- FINAL Swedish Code of Statutes Ordinance amending the Ordinance (1999:58) banning certain products SFS 2018:1587 that are harmful to health Published Issued on 4 October 2018 on 9 October 2018 The Government hereby lays down1 that the annex to the Ordinance (1999:58) prohibiting certain products that are harmful to health shall read as set out below. ___________ This ordinance shall enter into force on 12 November 2018. On behalf of the government ANNIKA STRANDHÄLL Kjell Rempler (Ministry of Health and Social Affairs) 1 See Directive (EU) 2015/1535 of the European Parliament and of the Council of 9 September 2015 laying down a procedure for the provision of information in the field of technical regulations and of rules on Information Society services. 2 Annex SFS 2018:1587 List of products to be regarded as products that are harmful to health in accordance with the Ordinance prohibiting certain products that are harmful to health N-methyl-1-(3,4-methylenedioxyphenyl)-2-butylamine (MBDB) 1-(3,4-methylenedioxyphenyl)-2-butylamine (BDB) 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DiPT) 5-methoxy-alphamethyltryptamine (5-MeO-AMT) 2,5-dimethoxy-4-ethylphenethylamine (2C-E) alpha-methyltryptamine (AMT) 2,5-dimethoxy-4-chlorophenethylamine (2C-C) 2,5-dimethoxy-4-methylphenethylamine (2C-D) 4-acetoxy-N,N-diisopropyltryptamine (4-AcO-DiPT) 4-hydroxy-N,N-diisopropyltryptamine (4-HO-DiPT) gamma-butyrolactone (GBL) 1,4-butanediol (1,4-BD) 4-acetoxy-N,N-methylisopropyltryptamine -

Appendix-2Final.Pdf 663.7 KB
North West ‘Through the Gate Substance Misuse Services’ Drug Testing Project Appendix 2 – Analytical methodologies Overview Urine samples were analysed using three methodologies. The first methodology (General Screen) was designed to cover a wide range of analytes (drugs) and was used for all analytes other than the synthetic cannabinoid receptor agonists (SCRAs). The analyte coverage included a broad range of commonly prescribed drugs including over the counter medications, commonly misused drugs and metabolites of many of the compounds too. This approach provided a very powerful drug screening tool to investigate drug use/misuse before and whilst in prison. The second methodology (SCRA Screen) was specifically designed for SCRAs and targets only those compounds. This was a very sensitive methodology with a method capability of sub 100pg/ml for over 600 SCRAs and their metabolites. Both methodologies utilised full scan high resolution accurate mass LCMS technologies that allowed a non-targeted approach to data acquisition and the ability to retrospectively review data. The non-targeted approach to data acquisition effectively means that the analyte coverage of the data acquisition was unlimited. The only limiting factors were related to the chemical nature of the analyte being looked for. The analyte must extract in the sample preparation process; it must chromatograph and it must ionise under the conditions used by the mass spectrometer interface. The final limiting factor was presence in the data processing database. The subsequent study of negative MDT samples across the North West and London and the South East used a GCMS methodology for anabolic steroids in addition to the General and SCRA screens. -

Alcohol and Drug Abuse Subchapter 9
Chapter 8 – Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority This rule is established under the authority of 18 V.S.A. §§ 4201 and 4202 which authorizes the Vermont Board of Health to designate regulated drugs for the protection of public health and safety. 2.0 Purpose This rule designates drugs and other chemical substances that are illegal or judged to be potentially fatal or harmful for human consumption unless prescribed and dispensed by a professional licensed to prescribe or dispense them and used in accordance with the prescription. The rule restricts the possession of certain drugs above a specified quantity. The rule also establishes benchmark unlawful dosages for certain drugs to provide a baseline for use by prosecutors to seek enhanced penalties for possession of higher quantities of the drug in accordance with multipliers found at 18 V.S.A. § 4234. 3.0 Definitions 3.1 “Analog” means one of a group of chemical components similar in structure but different with respect to elemental composition. It can differ in one or more atoms, functional groups or substructures, which are replaced with other atoms, groups or substructures. 3.2 “Benchmark Unlawful Dosage” means the quantity of a drug commonly consumed over a twenty-four-hour period for any therapeutic purpose, as established by the manufacturer of the drug. Benchmark Unlawful dosage is not a medical or pharmacologic concept with any implication for medical practice. Instead, it is a legal concept established only for the purpose of calculating penalties for improper sale, possession, or dispensing of drugs pursuant to 18 V.S.A. -

JWH-073 Critical Review Report Agenda Item 4.11
JWH-073 Critical Review Report Agenda item 4.11 Expert Committee on Drug Dependence Thirty-eight Meeting Geneva, 14-18 November 2016 38th ECDD (2016) Agenda item 4.11 JWH-073 Page 2 of 29 38th ECDD (2016) Agenda item 4.11 JWH-073 Contents Acknowledgements ................................................................................................................... 5 Summary ................................................................................................................................... 6 1. Substance identification .................................................................................................... 7 A. International Nonproprietary Name (INN) .................................................................. 7 B. Chemical Abstract Service (CAS) Registry Number .................................................. 7 C. Other Chemical Names ................................................................................................ 7 D. Trade Names ................................................................................................................ 7 E. Street Names ................................................................................................................ 7 F. Physical Appearance .................................................................................................... 7 G. WHO Review History ................................................................................................. 7 2. Chemistry .......................................................................................................................... -

Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority
Chapter 8 – Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority This rule is established under the authority of 18 V.S.A. §§ 4201 and 4202 which authorizes the Vermont Board of Health to designate regulated drugs for the protection of public health and safety. 2.0 Purpose This rule designates drugs and other chemical substances that are illegal or judged to be potentially fatal or harmful for human consumption unless prescribed and dispensed by a professional licensed to prescribe or dispense them, and used in accordance with the prescription. The rule restricts the possession of certain drugs above a specified quantity. The rule also establishes benchmark unlawful dosages for certain drugs to provide a baseline for use by prosecutors to seek enhanced penalties for possession of higher quantities of the drug in accordance with multipliers found at 18 V.S.A. § 4234. 3.0 Definitions 3.1 “Analog” means one of a group of chemical components similar in structure but different with respect to elemental composition. It can differ in one or more atoms, functional groups or substructures, which are replaced with other atoms, groups or substructures. 3.2 “Benchmark Unlawful Dosage” means the quantity of a drug commonly consumed over a twenty-four hour period for any therapeutic purpose, as established by the manufacturer of the drug. Benchmark Unlawful dosage is not a medical or pharmacologic concept with any implication for medical practice. Instead, it is a legal concept established only for the purpose of calculating penalties for improper sale, possession, or dispensing of drugs pursuant to 18 V.S.A. -

Postmortem Distribution of Mepirapim and Acetyl Fentanyl in Biological Fuid and Solid Tissue Specimens Measured by the Standard Addition Method
Forensic Toxicology https://doi.org/10.1007/s11419-018-0431-z ORIGINAL ARTICLE Postmortem distribution of mepirapim and acetyl fentanyl in biological fuid and solid tissue specimens measured by the standard addition method Akira Mochizuki1 · Hiroko Nakazawa1 · Noboru Adachi2 · Kenichi Takekawa1 · Hideki Shojo2 Received: 6 May 2018 / Accepted: 26 June 2018 © The Author(s) 2018 Abstract Purpose Mepirapim is a new synthetic cannabinoid. We previously reported that the concentrations of unchanged mepirapim in whole blood and urine were much higher than those of other synthetic cannabinoids. To determine the postmortem distri- bution of mepirapim and acetyl fentanyl in the deceased individual, we established a standard addition method for detailed analysis by liquid chromatography–mass spectrometry (LC–MS) for quantifcation of these drugs. Methods The LC–MS method was fully validated for linearity, extraction recovery, matrix efect and repeatability. Results Good linearities, extraction recoveries, matrix efects and repeatabilities were shown for both target compounds in all specimens. The concentrations of mepirapim and acetyl fentanyl in three body fuid specimens and 12 solid tissue speci- mens were measured. For mepirapim, the highest concentrations were found in the liver and kidney, and the concentrations in the blood and urine specimens were one order of magnitude lower than the high concentrations in the solid tissues except the psoas major muscle. For acetyl fentanyl, the highest concentrations were found in the myocardium, spleen and kidney, and the concentrations in the body fuid specimens were also one order of magnitude lower than the highest concentrations in the solid tissues. There were concentration diferences of mepirapim and acetyl fentanyl among the regions of the brain. -

Molecular Pharmacology of Synthetic Cannabinoids: Delineating CB1 Receptor-Mediated Cell Signaling
International Journal of Molecular Sciences Review Molecular Pharmacology of Synthetic Cannabinoids: Delineating CB1 Receptor-Mediated Cell Signaling Kenneth B. Walsh * and Haley K. Andersen Department of Pharmacology, Physiology & Neuroscience, University of South Carolina, School of Medicine, Columbia, SC 29208, USA; [email protected] * Correspondence: [email protected] Received: 24 July 2020; Accepted: 14 August 2020; Published: 25 August 2020 Abstract: Synthetic cannabinoids (SCs) are a class of new psychoactive substances (NPSs) that exhibit high affinity binding to the cannabinoid CB1 and CB2 receptors and display a pharmacological profile similar to the phytocannabinoid (-)-trans-D9-tetrahydrocannabinol (THC). SCs are marketed under brand names such as K2 and Spice and are popular drugs of abuse among male teenagers and young adults. Since their introduction in the early 2000s, SCs have grown in number and evolved in structural diversity to evade forensic detection and drug scheduling. In addition to their desirable euphoric and antinociceptive effects, SCs can cause severe toxicity including seizures, respiratory depression, cardiac arrhythmias, stroke and psychosis. Binding of SCs to the CB1 receptor, expressed in the central and peripheral nervous systems, stimulates pertussis toxin-sensitive G proteins (Gi/Go) resulting in the inhibition of adenylyl cyclase, a decreased opening of N-type Ca2+ channels and the activation of G protein-gated inward rectifier (GIRK) channels. This combination of signaling effects dampens neuronal activity in both CNS excitatory and inhibitory pathways by decreasing action potential formation and neurotransmitter release. Despite this knowledge, the relationship between the chemical structure of the SCs and their CB1 receptor-mediated molecular actions is not well understood. -

1 Councilmember Charles Allen 2 3 4 5 a BILL
1 ___________________________ 2 Councilmember Charles Allen 3 4 5 6 A BILL 7 8 _____ 9 10 11 IN THE COUNCIL OF THE DISTRICT OF COLUMBIA 12 13 __________ 14 15 16 To amend, on an emergency basis, due to congressional review, the District of Columbia 17 Uniform Controlled Substances Act of 1981 to add certain classes and substances to the 18 list of Schedule I controlled substances. 19 20 BE IT ENACTED BY THE COUNCIL OF THE DISTRICT OF COLUMBIA, That this 21 act may be cited as the “Revised Synthetics Abatement and Full Enforcement Drug Control 22 Congressional Review Emergency Amendment Act of 2018”. 23 Sec. 2. The District of Columbia Uniform Controlled Substances Act of 1981, 24 effective August 5, 1981 (D.C. Law 4-29; D.C. Official Code § 48-901.01 et seq.), is amended as 25 follows: 26 (a) Section 102(27) (D.C. Official Code § 48-901.02(27)) is amended as follows: 27 (1) Strike the phrase “as used in section 204(3) and section 206(1)(D)” and insert 28 the phrase “as used in section 204(3), (5), and (6) and section 206(1)(D)” in its place. 29 (2) Strike the phrase “As used in section 204(3)” and insert the phrase “As used in 30 section 204(3), (5), and (6)” in its place. 31 (b) Section 204 (D.C. Official Code § 48-902.04) is amended as follows: 32 (1) Paragraph (3) is amended as follows: 1 33 (A) The lead-in language is amended by striking the phrase “(for purposes 34 of this paragraph only, the term “isomer” includes the optical, position, and geometric isomers):” 35 and inserting a colon in its place. -

Of All the Abused Drugs out on the Market, Synthetic Marijuana Scares
370 SYNTHETIC CANNABINOIDS | 7x more than other labs’ SYNCBN panels Synthetic cannabinoids, also known as synthetic marijuana or a number of common names such as Of all the abused Spice, Kush, or K2, have been implicated in overdoses and fatalities nationwide. These drugs are designed to interact with the body’s cannabinoid receptors but are far more potent and cause harmful drugs out on the side effects. Users under the influence of these compounds exhibit behaviors ranging from hallucination, aggression, to paranoia. Unfortunately, lab analyses and legislation cannot keep up with the creativity of market, synthetic the underground labs who can produce new compounds with a change in structural chemistry. marijuana scares With Quality Forensic Toxicology’s new Comprehensive Synthetic Cannabinoid Panel, we put the forensic scientist back in front of rogue manufacturers. This new panel is designed to anticipate what’s me the most. coming to your city next. QFT has cutting edge analytical resources and experienced forensic scientists —Dr. Alexander Garrard to provide rapid and accurate analysis of blood specimens for the presence of these compounds. Using Clinical managing director Washington Poison Center quadrupole time-of-flight (Q-TOF) mass spectrometry, QFT has the capability to screen for hundreds of synthetic cannabinoid compounds, metabolites, and artifacts. COMPREHENSIVE SYNTHETIC CANNABINOID PANEL (±)-ORG 28611 ADB-FUBINACA, ADBICA HU-210, 211, 308, 331 Mepirapim 1-(4-Methoxyphenyl) piperazine ADB-PINACA IMMA MMB018, MMB2201 3,4-MDMA -
Download Product Insert (PDF)
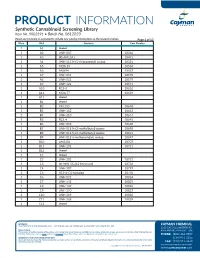
PRODUCT INFORMATION Synthetic Cannabinoid Screening Library Item No. 9002891 • Batch No. 0612023 Panels are routinely re-evaluated to include new catalog introductions as the research evolves. Page 1 of 13 Plate Well Contents Item Number 1 A1 Unused 1 A2 JWH 007 10266 1 A3 (R)-AM1241 10491 1 A4 JWH 018 N-(5-chloropentyl) analog 10521 1 A5 MDA 19 10563 1 A6 AM694 10567 1 A7 JWH 251 10578 1 A8 JWH 081 10579 1 A9 JWH 122 10591 1 A10 RCS-8 10636 1 A11 MDA 77 10639 1 A12 Unused 1 B1 Unused 1 B2 KM 233 10640 1 B3 JWH 182 10643 1 B4 JWH 210 10644 1 B5 RCS-4 10645 1 B6 JWH 098 10680 1 B7 JWH 018 N-(2-methylbutyl) isomer 10690 1 B8 JWH 018 N-(3-methylbutyl) isomer 10691 1 B9 JWH 018 6-methoxyindole analog 10697 1 B10 AM2201 10707 1 B11 JWH 201 10721 1 B12 Unused 1 C1 Unused 1 C2 JWH 302 10722 1 C3 (±)-WIN 55,212 (mesylate) 10736 1 C4 JWH 307 10797 1 C5 RCS-4-C4 homolog 10798 1 C6 JWH 031 10824 1 C7 JWH 145 10825 1 C8 JWH 147 10826 1 C9 JWH 370 10827 1 C10 JWH 369 10828 1 C11 JWH 368 10829 1 C12 Unused WARNING CAYMAN CHEMICAL THIS PRODUCT IS FOR RESEARCH ONLY - NOT FOR HUMAN OR VETERINARY DIAGNOSTIC OR THERAPEUTIC USE. 1180 EAST ELLSWORTH RD SAFETY DATA ANN ARBOR, MI 48108 · USA This material should be considered hazardous until further information becomes available. -

Dissertation Submitted As Part of the Requirement for the Bsc Forensic Science
Faculty of Science & Technology An assessment of the effects synthetic cannabinoids can have on a person’s ability to drive A dissertation submitted as part of the requirement for the BSc Forensic Science Ellis George May 2017 Abstract Cannabis is one of the most commonly used drugs for its euphoric effects but due to its psychotropic effects it has been categorised as a Class B drug. As cannabis became illegal many people turned to what they believed to be a safe legal alternative to cannabis. Because of this reason synthetic cannabinoids emerged into the market. These are man-made chemicals that mimic the effects of cannabis. Although they were classed as ‘legal’ they were not necessarily safe. As cannabis was one of the most common drugs used found in driving cases this dissertation aimed to look at synthetic cannabinoids and the effect they have on a persons’ ability to drive. To do this a series of monographs will describe the occurrence and usage, blood concentration, metabolism and excretion, toxicity and the use of synthetic cannabinoids in driving under the influence cases. Identifying the relevant specific synthetic cannabinoids in the literature and extracting the related information that is reported created these monographs. The information collected provides evidence that synthetic cannabinoids cause impairment. Using standardised field sobriety tests, clues were noted by drug recognition experts that indicated impairment for the suspects. Blood concentrations in the related cases were reported to suggest what kind of concentration could produce that level and kind of impairment. Each monograph produced, details the effects that the relevant synthetic cannabinoid has on impairment and the person’s ability to drive. -

King's Research Portal
King’s Research Portal DOI: 10.1515/pac-2017-0605 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Abbate, V., Schwenk, M., Presley, B., & Uchiyama, N. (2018). The ongoing challenge of novel psychoactive drugs of abuse. Part I. Synthetic cannabinoids (IUPAC Technical Report). PURE AND APPLIED CHEMISTRY, 90(8). https://doi.org/10.1515/pac-2017-0605 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim.