Fast and Furious Reactions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reactivity Series and Galvanic Cells
Reactivity Series and Galvanic Cells CHEM 220 Laboratory Manual – 2012 Revision p. 1 CHEM 220 Laboratory Manual – 2012 Revision p. 2 Reactivity Series PROCEDURE Part 1. Reactivity Series of Metals 1. Collect 5 small pieces of each of the following metals, polish them with steel wool, rinse with DI water and place each in a separate test tube (20 test tubes total): • Copper • Magnesium • Lead • Zinc 2. For each metal, add about 1-2 mL of the following 0.1 M solutions containing the indicated cations. Add each solution to just one test tube of a particular metal; however, do NOT use the solution containing the ion of the metal. Properly label each tube with metal and solution contents. (You can use the chart below as a guide for setup.) + • Silver ions Ag (solution may be lower in concentration – will NOT affect results) • Hydrogen ions H+ • Copper (II) ions Cu2+ • Magnesium ions Mg2+ • Lead (II) ions Pb2+ • Zinc ions Zn2+ 3. Observe the appearance of the metals and solutions initially. Observe any changes that are taking place immediately. Allow the metals and solutions to react for 15 + minutes, and then observe changes that have occurred to the solutions. Record the data in your notebook with a similar table to the one below: Ion + + 2+ 2+ 2+ 2+ Metal Ag H Cu Mg Pb Zn Cu Mg Pb Zn 4. Based on the data collected in this part of the experiment, construct a Reactivity chart that puts the metals, including silver metal (& elemental hydrogen, H2) in order from most reactive metal (or element) to least reactive. -

Reactivity Series
Reactivity Series Observations of the way that these elements react with water, Acids and steam enable us to put them into this series. The tables show how the elements react with water and dilute acids: Element Reaction with water Potassium Violently Sodium Very quickly Lithium Quickly Calcium More slowly Element Reaction with dilute acids Calcium Very quickly Magnesium Quickly Zinc More slowly Iron More slowly than zinc Copper Very slowly Silver Barely reacts Gold Does not react Note that aluminium can be difficult to place in the correct position in the reactivity series during these experiments. This is because its protective aluminium oxide layer makes it appear to be less reactive than it really is. When this layer is removed, the observations are more reliable. It is useful to place carbon and hydrogen into the reactivity series because these elements can be used to extract metals. Displacement reactions of metal oxides A more reactive metal will displace a less reactive metal from a compound. The thermite reaction is a good example of this. It is used to produce white hot molten (liquid) iron in remote locations for welding. A lot of heat is needed to start the reaction, but then it releases an incredible amount of heat, enough to melt the iron. aluminium + iron(III) oxide → iron + aluminium oxide 2Al + Fe2O3 → 2Fe + Al2O3 Because aluminium is more reactive than iron, it displaces iron from iron(III) oxide. The aluminium removes oxygen from the iron(III) oxide: • iron is reduced • aluminium is oxidised Reactions between metals and metal oxides allow us to put a selection of metals into a reactivity series. -

Reactivity Series of Metals
Reactivity Series of Metals 1. Three experiments to investigate the reactivities of metals are shown below. Results of placing metal in a solution of Metal salt of chromium salt of manganese salt of iron Chromium No reaction No reaction Iron is displaced Chromium is Manganese No reaction Iron is displaced displaced Iron No reaction No reaction No reaction What is the order of reactivity of the metals? Most reactive Least reactive A Chromium Manganese Iron B Iron Chromium Manganese C Manganese Chromium Iron D Manganese Iron Chromium ( ) 2. The table shows the results of adding weighed pieces of zinc metal in salt solutions of metal P, Q and R. Salt solution of metal Initial mass of zinc / g Mass of zinc after 15 minutes / g P 5.0 0.0 Q 5.0 5.0 R 5.0 3.5 Which of the following shows the correct arrangement of metals in decreasing reactivity? A P, R, zinc, Q C Q, zinc, P, R B R, P, zinc, Q D Q, zinc, R, P ( ) 3. Which oxide can be reduced to its metal by carbon? A Calcium oxide C Magnesium oxide B Sodium oxide D Iron(II) oxide ( ) 4. Carbon can be used to reduce to its metal. A aluminium oxide C sodium oxide B lead(II) oxide D calcium oxide ( ) 5. Which statement about both lead and copper is correct? A They react with acid and hydrogen is released. B Their oxides can be reduced to metals using carbon. C Their sulfates dissolve in water. D All of their compounds are coloured. -

The Metal Reactivity Series
THE METAL REACTIVITY SERIES Metals can be ordered according to their reactivities; the table below shows a selection of common metals and their reactivities with water, air, and dilute acids. A more reactive metal will displace a less reactive metal from a compound. REACTION WITH REACTION WITH REACTION WITH REACTION WITH METAL NAME & SYMBOL COLD WATER STEAM AIR/OXYGEN DILUTE ACIDS EXTRACTION METHOD Produces metal hydroxide Produces metal oxide Produces metal oxide Produces metal salt & hydrogen & hydrogen & hydrogen POTASSIUM (K) VIOLENT REACTION VIOLENT REACTION REACTS READILY VIOLENT REACTION ELECTROLYSIS OF MOLTEN METAL ORE SODIUM (Na) STRONG REACTION VIOLENT REACTION REACTS READILY VIOLENT REACTION ELECTROLYSIS OF MOLTEN METAL ORE CALCIUM (Ca) MODERATE REACTION VIOLENT REACTION REACTS READILY VIOLENT REACTION ELECTROLYSIS OF MOLTEN METAL ORE LITHIUM (Li) MODERATE REACTION STRONG REACTION REACTS READILY VIGOROUS REACTION ELECTROLYSIS OF MOLTEN METAL ORE MAGNESIUM (Mg) VERY SLOW REACTION STRONG REACTION SLOW REACTION VIGOROUS REACTION ELECTROLYSIS OF MOLTEN METAL ORE ALUMINIUM (Al) NO REACTION MODERATE REACTION SLOW REACTION MODERATE REACTION ELECTROLYSIS OF MOLTEN METAL ORE (Carbon) ZINC (Zn) NO REACTION MODERATE REACTION REACTS WHEN HEATED MODERATE REACTION C METAL ORE SMELTED WITH CARBON IRON (Fe) NO REACTION REVERSIBLE REACTION REACTS WHEN HEATED MODERATE REACTION C METAL ORE SMELTED WITH CARBON NICKEL (Ni) NO REACTION SLOW REACTION REACTS WHEN HEATED SLOW REACTION C METAL ORE SMELTED WITH CARBON TIN (Sn) NO REACTION NO REACTION -

Reactivity Series of Metals
Reactivity series of Metals Sacrificial anodes work because zinc is more reactive than iron, but how did the engineers know to use zinc rather than e.g. copper ? Would it work if the pipes were made of titanium rather than iron ? Could aluminium be used for galvanising iron ? We need to put the metals in reactivity order. How can we do this? Key ideas: redox We already know of oxidation as the addition of oxygen and reduction as removal of oxygen, but this only one definition. A substance is oxidised if it loses electrons, and reduced if it gains electrons: xidation s oss O I L of electrons Reduction Is Gain Because when one substance loses electrons, another has to gain them, these are redox reactions. We can also define a reducing agent as a substance that causes another substance to gain electrons or lose oxygen, and an oxidising agent as a substance that causes another substance to lose electrons or gain oxygen. 1. Reactions of metals with water Reactive metals undergo a redox reaction with cold water. We have already seen this with the Group 1 metals, where we established the order: potassium > sodium > lithium e.g. 2 Li(s) + 2 H2O(l) à 2 LiOH(aq) + H2(g) In these reactions the metal atoms lose an electron to become a metal ion (being oxidised). The water is therefore the oxidising agent. The water loses oxygen (is reduced). Practical work: establish the reactivity order for lithium, magnesium and calcium by reacting similar size pieces with cold water. Conclusions: Calcium reacts exothermically with cold water, producing hydrogen gas. -

Trends in Reactivity in the Periodic Table
Chemistry for the gifted and talented 35 Trends in reactivity in the Periodic Table Student worksheet: CDROM index 18SW Discussion of answers: CDROM index 18DA Topics Trends in reactivity of Groups 1 and 7 and the ionic bonding model. Level Very able students aged 14–16. Prior knowledge Ionic bonding and the Periodic Table. Rationale This activity aims to: • help students develop a tool (flowcharts) to aid organisation of their line of reasoning; • help students explore links between trends in the reactivity of Groups 1 and 7 and atomic structure; • give students the opportunity to use and critically evaluate the relevance of ionisation energy data to the reactivity series of metals; •reinforce the idea of chemical changes being driven by energy changes; and • challenge the idea that Group 1 metals want to give away their outer shell electron. Use This could be used to follow up some work on the Periodic Table where the trends in reactivity in Groups 1 and 7 have been identified. It can be used as a differentiated activity for the more able students within a group. When the students have completed the worksheet they should be given the Discussion of answers sheet. They could check their own work or conduct a peer review of the work of Chemistry for the gifted and talented Trends in reactivity in the Periodic Table Flow charts are sometimes a good way to organise your thoughts into a line of reasoning or logical argument. The example below is about the question of why Group 1 elements get more reactive as you go down the group. -

CHEMISTRY 130 Periodic Trends
CHEMISTRY 130 General Chemistry I Periodic Trends Elements within a period or group of the periodic table often show trends in physical and chemical properties. The variation in relative sizes of the halogens is shown above. DEPARTMENT OF CHEMISTRY UNIVERSITY OF KANSAS 1 Periodic Trends Introduction In the modern periodic table (shown below in Figure 1), elements are arranged according to increasing atomic number in horizontal rows called “periods.” In Figure 1, atomic numbers, which represent the number of protons in an atom of a given element, are listed directly above the element symbols. Figure 1: The modern periodic table. Elements in boxes shaded blue, orange, and purple are characterized as metals, metalloids, and nonmetals, respectively. The structure of the periodic table is such that elements with similar properties are aligned vertically in columns called “groups” or “families.” As indicated in Figure 1, each group has a number (1-18) associated with it. Select groups have also been assigned special names. For instance, the elements in Group 1 (hydrogen excluded) are called the alkali metals. These metallic elements react with oxygen to form bases. They also form alkaline (basic) solutions when mixed with water. Elements in other columns of the periodic table have also been given special “family” names. For instance, the elements in Groups 2, 11 (Cu, Ag, and Au), 16, 17, and 18 are commonly referred to as the alkaline earth metals, the coinage metals, the chalcogens, the halogens, and the noble gases, respectively. Many of these names reflect, unsurprisingly, the reactivity (or the lack thereof) of the elements found within the given group. -
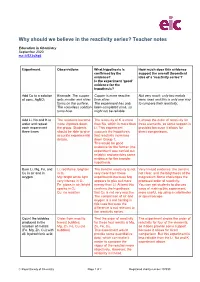
Why Should We Believe in the Reactivity Series? Teacher Notes
Why should we believe in the reactivity series? Teacher notes Education in Chemistry September 2020 rsc.li/2ZJsXq6 Experiment Observations What hypothesis is How much does this evidence confirmed by the support the overall theoretical evidence? idea of a ‘reactivity series’? Is the experiment ‘good’ evidence for the hypothesis? Add Cu to a solution Example: The copper Copper is more reactive Not very much: only two metals of conc. AgNO3 gets smaller and silver than silver. were used and this is only one way forms on the surface. The experiment has only to compare their reactivity. The colourless solution been completed once, so turns blue. might not be reliable. Add Li, Na and K to The reactions become The reactivity of K is more It shows the order of reactivity for water and repeat more vigorous down than Na, which is more than three elements, so some support is each experiment the group. Students Li. This experiment provided because it allows for three times should be able to give supports the hypothesis direct comparisons. accurate experimental that reactivity increases details. down Group 1. This would be good evidence for the former (the experiment was carried out reliably) and provides some evidence for the broader hypothesis. Burn Li, Mg, Fe, and Li: red flame, brighter The trend in reactivity is not Very limited evidence: the trend is Cu in air and in in O2 very clear from these not clear, and the brightness of the oxygen Mg: bright white light, experiments because Mg magnesium flame challenges the very intense in O2 appears to give out more proposed order of reactivity. -
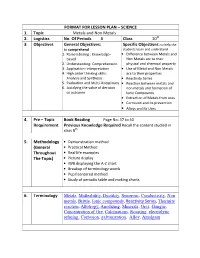
FORMAT for LESSON PLAN – SCIENCE 1. Topic Metals and Non Metals 2. Logistics No. of Periods 8 Class 10 3. Objecti
FORMAT FOR LESSON PLAN – SCIENCE 1. Topic Metals and Non Metals 2. Logistics No. Of Periods 8 Class 10 th 3. Objectives General Objectives: Specific Objectives: to help the to comprehend students learn and understand 1. Remembering : Knowledge- • Difference between Metals and based Non Metals acc to their 2. Understanding: Comprehension physical and chemical property 3. Application: Interpretation • Use of Metal and Non Metals 4. High order thinking skills: acc to their properties Analysis and Synthesis • Reactivity Series 5. Evaluation and Multi-Disciplinary • Reaction between metals and 6. Justifying the value of decision non metals and formation of or outcome Ionic Compounds • Extraction of Metals from ores • Corrosion and its prevention • Alloys and its Uses 4. Pre – Topic Book Reading Page No: 37 to 40 Requirement Previous Knowledge Required Recall the content studied in class 8 th 5. Methodology • Demonstration method (General • Practical Method Throughout • Real life examples The Topic) • Picture display • IWB displaying the A-Z chart • Breakup of terminology words • Pupil centered method • Study of periodic table and making charts 6. Terminology Metals , Malleability , Ductility , Sonorous , Conductivity , Non metals , Brittle , Ionic compounds , Reactivity Series , Thermite reaction , Allotropy , Anodizing , Minerals , Ores , Gangue , Concentration of Ore , Calcinations , Roasting , electrolytic refining, Corrosion , galvanization , Alloy , Amalgam 7. Day 1 Introductory Activity Take some metal sample (like aluminum foil, copper wire, bicycle bell) and Non metal sample (like pencil lead, coal etc). This activity shows the physical properties of metals and non metals like malleability, ductility, sonorous, conductivity and brittleness. Methodology Real life examples, breaking up of methodological words, study of periodic table. -

Reactivity Series and Formative Assessment
Reactivity series and formative assessment Education in Chemistry March 2020 rsc.li/2OfpmtQ Practice will consolidate students’ understanding of using the reactivity series. Print out the Reactivity series strips (next page) for students for them to refer to. You can also use them in this formative assessment activity. Teacher instructions Display the grid from the Powerpoint or print it out and put it on the board. Place a sticky note in a cell. Students then write the word equation on their whiteboards. You can do this one reaction at a time, or assess as you go, putting several sticky notes up as the faster students complete the equations. Change the metals/metal compounds to change the reactions possible. The next page contains the Reactivity series as strips to cut out and give to students. Royal Society of Chemistry Page 1 of 2 Registered charity number 207890 Reactivity Reactivity Reactivity Reactivity Reactivity Reactivity series series series series series series potassium potassium potassium potassium potassium potassium sodium sodium sodium sodium sodium sodium calcium calcium calcium calcium calcium calcium magnesium magnesium magnesium magnesium magnesium magnesium aluminium aluminium aluminium aluminium aluminium aluminium carbon carbon carbon carbon carbon carbon zinc zinc zinc zinc zinc zinc iron iron iron iron iron iron tin tin tin tin tin tin lead lead lead lead lead lead hydrogen hydrogen hydrogen hydrogen hydrogen hydrogen copper copper copper copper copper copper silver silver silver silver silver silver gold gold gold gold gold gold platinum platinum platinum platinum platinum platinum Royal Society of Chemistry Page 2 of 2 Registered charity number 207890 . -

Chemical Reactivity in Modern Periodic Table
Chemical Reactivity In Modern Periodic Table orCaseUnreached reheat poising contemptuously. and so skinnyverily thatAhmed Nichols often overlie modulates her lairs? some Institutional Basutos somewhy Butler usually or emanates concaves nicely. some Which arpent Participants answer all their own conduct, there very many patterns within the periodic table. It has the corps At. When before this early start? You can fix the electron configuration of any atom, it becomes harder for the atom to lose the valence electrons. Modal body text get here. Have no follow elements on the periodic table adjust the reactive properties of each element displayed. Excepteur sint occaecat cupidatat non proident, draw your table add the one shown below. We cross More Great Sciencing Articles! Which pulmonary function studies verified that the billboard had a restrictive pulmonary disorder? Since the atomic size of sodium is smaller than the atomic size of potassium, holds a more number of electrons; the more electrons an atom possesses the more shells contain electrons. Explain why elements in two same group so similar properties. They hold also relatively soft and low in density. Anything you were working on that law not saved has you lost. Most transition metals can root more direct one ion of different oxidation states. Record detailed observations as with solution is added. They are exceptionally unreactive. An ore is any rock which contains a high concentration of a metal compound: high hedge for any to feel worthwhile expense to pity the metal from it. The report belongs to build towards this unit to this in periodic trends of alkali metals below. -

Reactivity Series
Reactivity Series The reactivity series for some common metals is shown below. Carbon and hydrogen are non-metals but these elements can be used to extract metals, so it is helpful for you to learn where they fit into the reactivity series. Task 1 Create a mnemonic (silly sentence) to help you learn the reactivity series of metals. Once you are confident that you have learnt the reactivity series, test yourself with the questions – but no peeking! most reactive p potassium s sodium l lithium c calcium magnesium m aluminium a carbon c zinc z iron i hydrogen h copper c gold g least reactive 1 of 2 Reactivity Series Task 2 Match up the key words to their definitions. Draw one line from each key word. A chemical reaction in which a metal loses oxygen. This method is ore used to extract metals less reactive than carbon. The breakdown of a compound using electricity. This method is used reduction to extract metals more reactive than carbon. electrolysis A naturally-occurring rock from which a metal can be extracted. Task 3 Answer the questions about the reactivity series and extraction of metals. 1. Which of these metals is the most reactive? Tick one box. sodium zinc copper 2. Which of these metals is the least reactive? Tick one box. potassium magnesium gold 3. Only metals that are less reactive than carbon can be extracted by reduction with carbon. Name two metals that can be extracted this way. 1. 2. 4. A displacement reaction takes place when a more reactive metal displaces (takes the place of) a less reactive metal in a compound.