(P-Phenylene Terephthalamide) Fibers During Heat Treatment Process: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Controlled/Living Radical Polymerization in Aqueous Media: Homogeneous and Heterogeneous Systems
Prog. Polym. Sci. 26 =2001) 2083±2134 www.elsevier.com/locate/ppolysci Controlled/living radical polymerization in aqueous media: homogeneous and heterogeneous systems Jian Qiua, Bernadette Charleuxb,*, KrzysztofMatyjaszewski a aDepartment of Chemistry, Center for Macromolecular Engineering, Carnegie Mellon University, 4400 Fifth Avenue, Pittsburgh, PA 15213, USA bLaboratoire de Chimie MacromoleÂculaire, Unite Mixte associeÂe au CNRS, UMR 7610, Universite Pierre et Marie Curie, Tour 44, 1er eÂtage, 4, Place Jussieu, 75252 Paris cedex 05, France Received 27 July 2001; accepted 30 August 2001 Abstract Controlled/living radical polymerizations carried out in the presence ofwater have been examined. These aqueous systems include both the homogeneous solutions and the various heterogeneous media, namely disper- sion, suspension, emulsion and miniemulsion. Among them, the most common methods allowing control ofthe radical polymerization, such as nitroxide-mediated polymerization, atom transfer radical polymerization and reversible transfer, are presented in detail. q 2001 Elsevier Science Ltd. All rights reserved. Keywords: Aqueous solution; Suspension; Emulsion; Miniemulsion; Nitroxide; Atom transfer radical polymerization; Reversible transfer; Reversible addition-fragmentation transfer Contents 1. Introduction ..................................................................2084 2. General aspects ofconventional radical polymerization in aqueous media .....................2089 2.1. Homogeneous polymerization .................................................2089 -

Poly(Sodium Acrylate)-Based Antibacterial Nanocomposite Materials
Poly(Sodium Acrylate)-Based Antibacterial Nanocomposite Materials Samaneh Khanlari Thesis submitted to the Faculty of Graduate and Postdoctoral Studies in partial fulfillment of the requirements for the degree of Doctorate in Philosophy in Chemical Engineering Department of Chemical and Biological Engineering Faculty of Engineering UNIVERSITY OF OTTAWA © Samaneh Khanlari, Ottawa, Canada, 2015 i ii Abstract Polymer-based bioadhesives for sutureless surgery provide a promising alternative to conventional suturing. In this project, a new poly(sodium acrylate)-based nanocomposite with antibacterial properties was developed. Poly(sodium acrylate), was prepared using a redox solution polymerization at room temperature; this polymer served as a basis for a nanocomposite bioadhesive material using silver nanoparticles. In-situ polymerization was chosen as a nanocomposite synthesizing method and three methods were applied to quantify the distribution and loadings of nanofiller in the polymer matrices. These included the Voronoi Diagram, Euclidean Minimum Spanning Tree (EMST) method and pixel counting. Results showed that pixel counting combined with the EMST method would be most appropriate for nanocomposite morphology quantification. Real-time monitoring of the in-situ polymerization of poly(sodium acrylate)- based nanocomposite was investigated using in-line Attenuated Total Reflectance/Fourier Transform infrared (ATR-FTIR) technique. The ATR-FTIR spectroscopy method was shown to be valid in reaction conversion monitoring using a partial least squares (PLS) multivariate calibration method and the results were consistent with the data from off-line water removal gravimetric monitoring technique. Finally, a second, more degradable polymer (i.e., gelatin and poly(vinyl alcohol)) was used to modify the degradation rate and hydrophilicity of the nanocomposite bioadhesive. -

Synthesis and Characterization of Solution and Melt Processible Poly(Acrylonitrile-Co-Methyl Acrylate) Statistical Copolymers
SYNTHESIS AND CHARACTERIZATION OF SOLUTION AND MELT PROCESSIBLE POLY(ACRYLONITRILE-CO-METHYL ACRYLATE) STATISTICAL COPOLYMERS Padmapriya Pisipati Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Macromolecular Science and Engineering James E. McGrath, Chair Judy S. Riffle, Acting Chair Eugene G. Joseph Sue J. Mecham Donald G. Baird 02/20/2015 Blacksburg, VA Keywords: melt processability, polyacrylonitrile, carbon fibers, methyl acrylate, acrylic fibers, plasticizer, solution processing, high tensile modulus Copyright 2015, Padmapriya Pisipati SYNTHESIS AND CHARACTERIZATION OF SOLUTION AND MELT PROCESSIBLE POLY (ACRYLONITRILE-CO-METHYL ACRYLATE) STATISTICAL COPOLYMERS ABSTRACT Padmapriya Pisipati Polyacrylonitrile (PAN) and its copolymers are used in a wide variety of applications ranging from textiles to purification membranes, packaging material and carbon fiber precursors. High performance polyacrylonitrile copolymer fiber is the most dominant precursor for carbon fibers. Synthesis of very high molecular weight poly(acrylonitrile-co-methyl acrylate) copolymers with weight average molecular weights of at least 1.7 million g/mole were synthesized on a laboratory scale using low temperature, emulsion copolymerization in a closed pressure reactor. Single filaments were spun via hybrid dry-jet gel solution spinning. These very high molecular weight copolymers produced precursor fibers with tensile strengths averaging 954 MPa with an elastic modulus of 15.9 GPa (N = 296). The small filament diameters were approximately 5 µm. Results indicated that the low filament diameter that was achieved with a high draw ratio, combined with the hybrid dry-jet gel spinning process lead to an exponential enhancement of the tensile properties of these fibers. -

Polymer Chemistry
CLOTHWORKERS LIBRARY UNIVERSITY OF LEEDS THERMAL AND OXIDATIVE DEGRADATION OF AN AROMATIC POLYAMIDE. by «. Shojan Nanoobhai Patel 2. Submitted in accordance with the requirement for the degree of Doctor of Philosophy under the supervision of Professor I.E. McIntyre and Dr. S.A. Sills. The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to work of others. Department of Textile Industries, The University of Leeds, Leeds, LS2 9JT. December 1992. CLASS MARK T:; ~S 2tC\4 -1- ABSTRACT. The thermal oxidation of an aromatic polyamide fibre, namel\' poly(m-xylylene adipamide) has been investigated. The volatile and non-volatile products of oxidation identified using T.L.C., H.P.L.C. and G.C.-M.S. include homologous series of monocarboxylic acids, dicarboxylic acids, n-alkyl amines, diamines and aldehydes. Furthermore, several aromatics, ketones and amides were also identified. Mechanisms for their derivation have been proposed which are based on oxidation reactions already established in polymer chemistry. These include a mechanism of ~ scission by an alkoxy radical thought to be responsible for the creation of the homologous series. MXD,6 fibres were also spun incorporating 100, 200 and 400ppm of a cobalt catalyst and the effect of the metal ions on the oxygen uptake of the fibres was investigated. The results show a consistent increase in the rate of oxygen uptake as the concentration of the cobalt is increased. Furthermore, T.G.A. oxidative degradation studies, conducted at isothermal temperatures in the solid state, show decreases in the activation energies associated with fibre oxidation with increased cobalt concentrations, implying the cobalt is a catalyst for oxidation. -

Polybenzimidazole Synthesis from Compounds with Sulfonate Ester Linkages
AN ABSTRACT OF THE THESIS OF Turgav Seckin for the degree of Master of Science in Chemistry presented on August 26, 1987 TITLE : Polybenzimidazole Synthesis Frog Comnounds with Sulfonate Ester Linkages Redacted for privacy Abstract approved : Dr. R.W. THIES This investigation of polybenzimidazole synthesis involvedthe two-stage low temperature solution polymerization of dialdehydes, One-step polycondensation of dialdehydes,two-stage melt polyconden- sation of diesters, and one-step solutionpolycondensation of diesters with 3,3'-diaminobenzidine. Dialdehyde or diester monomers were prepared which contained aromatic units whichwere hooked toget- her with sulfonate linkages. Polymerization of these monomers with 3,3'-diaminobenzidine resulted in moderate viscosities of0.4-0.8 dlg in DMSO at 19°C. The polybenzimidazoles obtained appear to be linear; most of them when tested immediatelyafter preparation, were largely soluble in certain acidicor dipolar approtic solvents. Films, obtained by dissolving the polymerin dipolar approtic solvents such as N,N-dimethylacetamideor N,N-dimethyl formamide, were flexible, clear, yellow to brown in color, and foldable depen- ding upon which monomers and which polymerization methodswere used. Polybenzimidazole Synthesis From Compounds with Sulfonate Ester Linkages by Turgay Seckin A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Completed August 26, 1987 Commencement June 1988 APPROVED Redacted for privacy Professor of Chemistry in Charge ofMajor Redacted for privacy Chairman of Department of Chemistry Redacted for privacy Dean of G ad ate School Date thesis is prepared August 17, 1987 Thesis typed by Turgay Seckin To my family for their never failing love andsupport NE MUTLU TURKUM DIYENE ACKNOWLEDGEMENT I would like to thank Prof. -

Temperature and Humidity Aging of Poly(P-Phenylene-2,6
Polymer Degradation and Stability 92 (2007) 1234e1246 www.elsevier.com/locate/polydegstab Temperature and humidity aging of poly( p-phenylene-2,6- benzobisoxazole) fibers: Chemical and physical characterization Joannie Chin a,*, Amanda Forster b, Cyril Clerici a, Lipiin Sung a, Mounira Oudina a, Kirk Rice b a Polymeric Materials Group, National Institute of Standards and Technology, Gaithersburg, MD 20899, United States b Office of Law Enforcement Standards, National Institute of Standards and Technology, Gaithersburg, MD 20899, United States Received 9 February 2007; received in revised form 20 March 2007; accepted 23 March 2007 Available online 19 April 2007 Abstract In recent years, poly( p-phenylene-2,6-benzobisoxazole) (PBO) fibers have become prominent in high strength applications such as body ar- mor, ropes and cables, and recreational equipment. The objectives of this study were to expose woven PBO body armor panels to elevated tem- perature and moisture, and to analyze the chemical, morphological and mechanical changes in PBO yarns extracted from the panels. A 30% decrease in yarn tensile strength, which was correlated to changes in the infrared peak absorbance of key functional groups in the PBO structure, was observed during the 26 week elevated temperature/elevated moisture aging period. Substantial changes in chemical structure were observed via infrared spectroscopy, as well as changes in polymer morphology using microscopy and neutron scattering. When the panels were removed to an ultra-dry environment for storage for 47 weeks, no further decreases in tensile strength degradation were observed. In a follow-on study, fibers were sealed in argon-filled glass tubes and exposed to elevated temperature; less than a 4% decrease in tensile strength was observed after 30 weeks, demonstrating that moisture is a key factor in the degradation of these fibers. -

1 050929 Quiz 1 Introduction to Polymers 1) in Class We Discussed
050929 Quiz 1 Introduction to Polymers 1) In class we discussed a hierarchical categorization of the terms: macromolecule, polymer and plastics, in that plastics are polymers and macromolecules and polymers are macromolecules; but all macromolecules are not polymers and all polymers are not plastics. a) Give an example of a macromolecule that is not a polymer. b) Give an example of a polymer that is not a plastic. c) Define polymer. d) Define plastic. e) Define macromolecule. 2) In class we observed a simulation of a small polymer chain of 40 flexible steps. The chain was observed to explore configurational space. a) Explain what the phrase "explore configurational space" means. b) Consider the two ends of the chain to be tethered with a separation distance of about 6 extended steps. If temperature were increased would the chain ends push apart or pull together the tether points? Explain why. c) If one chain end is fixed in Cartesian space at x = 0, what are the limits on the x-axis for the other chain end as it explores configurational space? d) What is the average value of the position on the x-axis? dγ 3) Polymer melts are processed at high rates of strain, , in an extruder, injection molder, or dt calander. a) Sketch a typical plot of polymer viscosity versus rate of strain on a log-log plot showing the power-law region and the Newtonian plateau at low rates of strain. b) Explain using this plot why polymers are processed at high rates of strain. c) Using your own words propose a molecular model that could explain shear thinning in polymers. -

Solution Polymerization of Acrylic Acid Initiated by Redox Couple Na-PS/Na-MBS: Kinetic Model and Transition to Continuous Process
processes Article Solution Polymerization of Acrylic Acid Initiated by Redox Couple Na-PS/Na-MBS: Kinetic Model and Transition to Continuous Process Federico Florit , Paola Rodrigues Bassam , Alberto Cesana and Giuseppe Storti * Politecnico di Milano, Dipartimento di Chimica, Materiali e Ingegneria Chimica “G. Natta”, Piazza Leonardo da Vinci, 32, 20133 Milano, Italy; federico.fl[email protected] (F.F.); [email protected] (P.R.B.); [email protected] (A.C.) * Correspondence: [email protected] Received: 28 May 2020; Accepted: 14 July 2020; Published: 16 July 2020 Abstract: This work aims at modeling in detail the polymerization of non-ionized acrylic acid in aqueous solution. The population balances required to evaluate the main average properties of molecular weight were solved by the method of moments. The polymerization process considered is initiated by a persulfate/metabisulfate redox couple and, in particular, the kinetic scheme considers the possible formation of mid-chain radicals and transfer reactions. The proposed model is validated using experimental data collected in a laboratory-scale discontinuous reactor. The developed kinetic model is then used to intensify the discontinuous process by shifting it to a continuous one based on a tubular reactor with intermediate feeds. One of the experimental runs is selected to show how the proposed model can be used to assess the transition from batch to continuous process and allow faster scale-up to industrial scale using a literature approach. Keywords: Poly(acrylic acid); free-radical polymerization; reaction model; process intensification; semi-batch to continuous 1. Introduction Poly(acrylic acid) (PAA) is a widely produced polymer with a growing, global annual production of 1.58 109 kg as of 2008 [1] and with applications in many industrial sectors. -
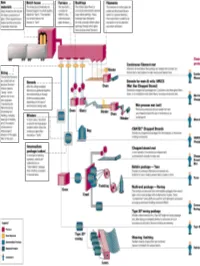
All About Fibers
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways. -

Emulsion Polymerization
Emulsion Polymerization Reference: Aspen Polymers: Unit Operations and Reaction Models, Aspen Technology, Inc., 2013. 1. Introduction Emulsion polymerization is an industrially important process for the production of polymers used as synthetic rubber, adhesives, paints, inks, coatings, etc. The polymerization is usually carried out using water as the dispersion medium. This makes emulsion polymerization less detrimental to the environment than other processes in which volatile organic liquids are used as a medium. In addition, emulsion polymerization offers distinct processing advantages for the production of polymers. Unlike in bulk or solution polymerization, the viscosity of the reaction mixture does not increase as dramatically as polymerization progresses. For this reason, the emulsion polymerization process offers excellent heat transfer and good temperature throughout the course of polymer synthesis. In emulsion polymerization, free-radical propagation reactions take place in particles isolated from each other by the intervening dispersion medium. This reduces termination rates, giving high polymerization rates, and simultaneously makes it possible to produce high molecular weight polymers. One can increase the rate of polymerization without reducing the molecular weight of the polymer. Emulsion polymerization has more recently become important for the production of a wide variety of specialty polymers. This process is always chosen when the polymer product is used in latex form. 2. Reaction Kinetic Scheme To appreciate the complexities of emulsion polymerization, a basic understanding of the fundamentals of particle formation and of the kinetics of the subsequent particle growth stage is required. A number of mechanisms have been proposed for particle formation. It is generally accepted that any one of the mechanisms could be responsible for particle formation depending on the nature of the monomer and the amount of emulsifier used in the recipe. -

Living Free Radical Polymerization with Reversible Addition – Fragmentation
Polymer International Polym Int 49:993±1001 (2000) Living free radical polymerization with reversible addition – fragmentation chain transfer (the life of RAFT) Graeme Moad,* John Chiefari, (Bill) YK Chong, Julia Krstina, Roshan TA Mayadunne, Almar Postma, Ezio Rizzardo and San H Thang CSIRO Molecular Science, Bag 10, Clayton South 3169, Victoria, Australia Abstract: Free radical polymerization with reversible addition±fragmentation chain transfer (RAFT polymerization) is discussed with a view to answering the following questions: (a) How living is RAFT polymerization? (b) What controls the activity of thiocarbonylthio compounds in RAFT polymeriza- tion? (c) How do rates of polymerization differ from those of conventional radical polymerization? (d) Can RAFT agents be used in emulsion polymerization? Retardation, observed when high concentra- tions of certain RAFT agents are used and in the early stages of emulsion polymerization, and how to overcome it by appropriate choice of reaction conditions, are considered in detail. Examples of the use of thiocarbonylthio RAFT agents in emulsion and miniemulsion polymerization are provided. # 2000 Society of Chemical Industry Keywords: living polymerization; controlled polymerization; radical polymerization; dithioester; trithiocarbonate; transfer agent; RAFT; star; block; emulsion INTRODUCTION carbonylthio compounds 2 by addressing the following In recent years, considerable effort1,2 has been issues: expended to develop free radical processes that display (a) How living is RAFT polymerization? -

Cryogenic Temperature Effects on the Mechanical Properties
CRYOGENIC TEMPERATURE EFFECTS ON THE MECHANICAL PROPERTIES OF CARBON, ARAMID, AND PBO FIBERS By William Chad Hastings A Thesis Submitted to the Faculty of Mississippi State University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Mechanical Engineering in the Department of Mechanical Engineering Mississippi State, Mississippi May 2008 CRYOGENIC TEMPERATURE EFFECTS ON THE MECHANICAL PROPERTIES OF CARBON, ARAMID, AND PBO FIBERS By William Chad Hastings Approved: ______________________________ ______________________________ Judy A. Schneider Anthony J. Vizzini Associate Professor of Mechanical Professor and Head of Aerospace Engineering Engineering Department (Director of Thesis) (Committee Member) ______________________________ ______________________________ Thomas E. Lacy Steve Daniewicz Associate Professor of Aerospace Professor and Graduate Coordinator Engineering of Mechanical Engineering (Committee Member) Department ______________________________ W.G. Steele Interim Dean and Professor Bagley College of Engineering Name: William Chad Hastings Date of Degree: May 2, 2008 Institution: Mississippi State University Major Field: Mechanical Engineering Major Professor: Judy Schneider Title of Study: CRYOGENIC TEMPERATURE EFFECTS ON THE MECHANICAL PROPERTIES OF CARBON, ARAMID, AND PBO FIBERS Pages in Study: 39 Candidate for Degree of Master of Science This study examines the effects of cryogenic temperatures on the mechanical properties of carbon, aramid, and poly(p-phenylene-2, 6-benzobisoxazole) (PBO) fibers. Although the mechanical properties are documented for these fibers at ambient and elevated temperatures, there is an absence of data in the open literature for how these fibers behave at very low temperatures. To evaluate the mechanical properties, the ASTM standard method for testing at ambient temperature was used as a baseline. The low temperature tests were conducted inside a double walled cryogenic chamber to evaluate the fiber performance at 100K.