Oxygenation of Turbine Discharges from Fort Patrick Henry Dam
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TENNESSEE FISH and WILDLIFE COMMISSION PROCLAMATION 15-27 SPORT FISHING Page1of18 Pursuant to the Authority Granted by Title
Page1of18 TENNESSEE FISH AND WILDLIFE COMMISSION PROCLAMATION 15-27 SPORT FISHING Pursuant to the authority granted by Title 70, Tennessee Code Annotated, and Sections 70-4-107 and 70- 4-119, thereof, the Tennessee Fish and Wildlife Commission proclaims the following regulations effective March 1, 2016. SECTION I. ENDANGERED SPECIES, GENERAL SEASONS, AND STATEWIDE CREEL, POSSESSION, AND LENGTH LIMITS. A. ENDANGERED SPECIES All fish identified as endangered or threatened or listed as in need of management as proclaimed by the Tennessee Fish and Wildlife Commission may not be taken. B. GAME FISH SPECIES The season is open year-round on the following species, unless otherwise specified in this proclamation. The possession limit is twice the daily creel limit. Only the daily creel limit may be possessed while afield. It shall also be unlawful to possess while afield any fish, which has been altered to the extent that its species and/or total body length cannot be determined. The length of a fish shall be determined with the fish laying on a flat ruler, the mouth closed, and the caudal (tail) fin lobes squeezed so as to produce the maximum length. The mouth of the fish may not be manipulated or extended. Unless stated otherwise a slot limit is a protected length range within which no fish may be harvested. Statewide daily creel and length limits for fish species are listed in the table below which includes exceptions for specific waterbodies. Additional exceptions are listed in Sections Ill, IV, V, and VI. See Special Definitions (Section XV) for reservoir boundary and specific area descriptions. -

2010 South Fork Holston River Environmental Monitoring Studies
2010 South Fork Holston River Environmental Monitoring Studies Patrick Center for Environmental Research 2010 South Fork Holston River Environmental Monitoring Studies Report No. 10-04F Submitted to: Eastman Chemical Company Tennessee Operations Submitted by: Patrick Center for Environmental Research 1900 Benjamin Franklin Parkway Philadelphia, PA 19103-1195 April 20, 2012 Executive Summary he 2010 study was the seventh in a series of comprehensive studies of aquatic biota and Twater chemistry conducted by the Academy of Natural Sciences of Drexel University in the vicinity of Kingsport, TN. Previous studies were conducted in 1965, 1967 (cursory study, primarily focusing on al- gae), 1974, 1977, 1980, 1990 and 1997. Elements of the 2010 study included analysis of land cover, basic environmental water chemistry, attached algae and aquatic macrophytes, aquatic insects, non-insect macroinvertebrates, and fish. For each study element, field samples were collected and analyzed from Scientists from the Academy's Patrick Center for Environmental Research zones located on the South Fork Holston River have conducted seven major environmental monitoring studies on the (Zones 2, 3 and 5), Big Sluice (Zone 4), mainstem South Fork Holston River since 1965. Holston River (Zone 6), and Horse Creek (Zones HC1and HC2), the approximate locations of which are shown below. The design of the 2010 study was very similar to that of previous surveys, allowing comparisons among surveys. In addition, two areas of potential local impacts were assessed for the first time: Big Tree Spring (BTS, located on the South Fork within Zone 2) and Kit Bottom (KU and KL in the Big Sluice, upstream of Zone 4). -

FORT PATRICK HENRY RESERVOIR Volume IV
FORT PATRICK HENRY RESERVOIR FINAL RESERVOIR LAND MANAGEMENT PLAN Volume IV NORTHEASTERN TRIBUTARY RESERVOIRS LAND MANAGEMENT PLAN FINAL ENVIRONMENTAL IMPACT STATEMENT MARCH 2010 This page intentionally left blank Document Type: EIS-Administrative Record Index Field: Final Environmental Document Project Name: Northeastern Tributary Reservoirs Land Plan Project Number: 2008-32 NORTHEASTERN TRIBUTARY RESERVOIRS LAND MANAGEMENT PLAN AND ENVIRONMENTAL IMPACT STATEMENT VOLUME IV Fort Patrick Henry Reservoir PREPARED BY: TENNESSEE VALLEY AUTHORITY MARCH 2010 For information, contact: Tennessee Valley Authority Holston-Cherokee-Douglas Land Planning Team 106 Tri-Cities Business Park Drive Gray, Tennessee 37615 Phone: (423) 467-3801 Fax: (423) 467-3815 Page intentionally blank Contents TABLE OF CONTENTS 1.0 INTRODUCTION ................................................................................................................... IV-1 1.1. Background ......................................................................................................................... IV-1 1.2. Purpose ............................................................................................................................... IV-2 2.0 PLANNING PROCESS ......................................................................................................... IV-5 2.1. Planning Goals .................................................................................................................. IV-10 2.2. Allocation Process ............................................................................................................ -

Tennessee Fishing Length Range, Only One Fish Over 20 Inches May Sunrise to One-Half Hour After Sunset License and Trout Stamp
Fishing Regulations Effective March 1, 2010 through February 28, 2011 Tennessee 2010 ishing F G U I D E Fis F Ju R hi ee N N e 12, g2010 Day Tennessee Wildlife Resources Agency www.tnwildlife.org Tailwater Brook Trout — New Live Bait Regulations f e at u r e 6 DowN FRom The mountaiNs For decades Tennessee’s anglers have climbed cascading mountain streams to catch brook trout. Nothing can replace the solitude of high-country fishing or the tug of these elegant fish on a lightweight rod. But after you have caught your 20th or so 5-inch brook trout, you may start to wonder “are there bigger brookies anywhere?” 6 CON t e N t S 2 whaT’s New FoR 2010 28 TRouT sTockiNg scheDuLe 4 TwRa oFFices 29 contamiNants iN Fish 4 TeNNessee wiLDLiFe 30 ReguLaTioNs FoR meThoDs ResouRces commissioN oTheR ThaN RoD & ReeL 8–9 LiceNse iNFoRmaTioN 32–34 IdentiFyiNg youR Fish 10 geNeRaL iNFoRmaTioN 36–37 TeNNessee aNgLeR RecogNiTioN pRogRam 11–13 LimiTs & ReguLaTioNs 37 QuesTioNs & aNsweRs 14–15 Live BaiT 38–39 StaTe RecoRDs 16–17 TwRa FishiNg Lakes 40–41 SportiNg showcase 18–23 ReseRvoiR ReguLaTioNs 26–27 TRouT ReguLaTioNs New 2010 regulations Tennessee 2010 ishing statewide F G U I D E • Several new regulations that limit the sale, use, and collection of live bait are listed in detail on pages 14–15. • Sport anglers must mark or tag their trotlines, limblines, and jugs with either their name and address or their current TWRA identification number. Region 3 Caney FoRk RiveR: Center Hill Dam downstream to Cumberland River, including tributaries Ε Rainbow, Brown, and Brook trout: creel limit 5 per day in combination. -
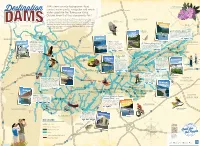
TVA's Dams Provide Hydropower, Flood Control, Water Quality, Navigation
TVA’s dams provide hydropower, ood Catawba Rhododendron (Rhododendron catawbiense) control, water quality, navigation and ample Lexington Destination water supply for the Tennessee Valley. Did you know that they also provide fun? Come summer, TVA operates its dams to ll the reservoirs for recreation. Boating, shing, swimming, rafting and blueway paddling are all supported Bald Eagle KENTUCKY by TVA with boat ramps, swim beaches and put ins. There are plenty of hiking (Haliaeetus leucocephalus) and biking trails, picnic pavilions, playgrounds, campsites, scenic overlooks SOUTH and other day-use areas, too. So plan a TVA vacation this year—you’re sure HO W.V. ILLINOIS LSTON 77 to have a dam good time. o R i v i e Rainbow Trout South Holston Dam - 1951 h r (Oncorhynchus mykiss) Because of its depth and clarity, South Holston Lake is a O FORT premier destination for inland scuba diving. The aerating Paducah PATRICK weir below the dam has many benets—among them NRY creating an oxygen-rich environment that’s fostered a HE world-class trout shery. ORRIS 75 MISSOURI N Hopkinsville 65 Kentucky – 1944 24 Norris - 1936 CKY Norris Dam—the rst built by a newly VIRGINIA KENTU Around Kentucky Lake there are Ft. Patrick Henry - 1953 55 formed TVA—is known for its many Fort Patrick Henry Dam is an ideal shing over 12,000 acres of state wildlife hiking and biking trails. The Norris River management areas, that offer small destination. The reservoir is stocked with rainbow Bluff Trail is a must-see destination for trout each year, and is also good for hooking and large game and waterfowl wildower enthusiasts each spring. -

Report to the Office of Management and Budget on the U.S. Fish And
Report to the Office of Management and Budget on the U.S. Fish and Wildlife Service's Fisheries Mitigation Programs Department of the Interior U.S. Fish and Wildlife Service May 28,2002 Table of Contents Introduction Definition of Terms General Authorities Recommendations to Facilitate Full Cost Recovery Recommendations for Administrative Actions Recommendations for Legislation Current Fisheries Mitigation Programs Overview Description of Fisheries Mitigation Programs Bureau of Reclamation U.S. Army Corps of Engineers Tennessee Valley Authority National Marine Fisheries Service Bonneville Power Administration Bureau of Indian Affairs U.S. Department of Agriculture Appendix I Recent Directives and Recommendations for Fisheries Mitigation Programs 75 Appendix I1 List of Hatcheries involved in Mitigation and State Location 77 Appendix I11 List of Species Raised for Mitigation Programs 78 INTRODUCTION The predecessor of the U.S. Fish and Wildlife Service (Service), the U.S. Commission on Fish and Fisheries, was formed in 1871 to protect and enhance fisheries which were being depleted by a growing Nation. Since that time, the Service's Fisheries Program, including the National Fish Hatchery System (NFHS), has been involved in, among other things, mitigating the losses of fish habitats, fish populations, and fishing opportunities. The U.S. Congress and the Courts have provided laws, appropriations, and orders which have authorized the construction and operation of dams, and other water diversion projects. The construction and operation of these Federal water resource development projects have had impacts on many water systems and their respective fish populations. The construction and operation of these projects have resulted in the need to build and operate fish hatcheries to provide direct mitigation (e.g., provide disease-free fish eggs or fish for stocking) for waters impaired by Federal water resource development projects. -

TVA Reservoir Aeration Diffuser System
Technical Paper 97-3 Presented at WaterPower ‘97 August 5-8, 1997, Atlanta, Georgia TVA Reservoir Aeration Diffuser System Mark H. Mobley1 Abstract The Tennessee Valley Authority (TVA) has developed an efficient and economical aeration diffuser design that has been installed and operated successfully at six TVA hydropower projects, one TVA nuclear plant and two non-power reservoirs. The line diffuser design transfers oxygen efficiently, and minimizes temperature destratification and sediment disruption by spreading the gas bubbles over a very large area in the reservoir. TVA test results have consistently indicated oxygen transfer efficiencies of 90 to 95 percent. The line diffusers are installed from the surface and can be retrieved if necessary for maintenance without the use of divers. The diffusers can be supplied with air or with oxygen, either from a bulk liquid oxygen storage tank, an onsite air separation plant, or air compressors. A line diffuser system can be designed to continuously aerate a large volume in the reservoir to handle peaking hydroturbine flows. Aeration within the reservoir can be an economical means to meet dissolved oxygen requirements for hydropower releases. Introduction The dissolved oxygen content of reservoir releases has become a problem for hydropower projects. Many FERC licenses now include minimum dissolved oxygen requirements. TVA has just completed the Lake Improvement Plan, a five- year, voluntary program that included developing and installing equipment to meet target levels of dissolved oxygen and minimum flow at 16 TVA hydropower projects. TVA now maintains target levels of dissolved oxygen and minimum flow at all of its 28 hydropower projects. -

Water Resources Data Tennessee Water Year 2004
U.S. Department of the Interior U.S. Geological Survey Water Resources Data Tennessee Water Year 2004 By D.F. Flohr, J.W. Garrett, J.T. Hamilton, and T.D. Phillips Water-Data Report TN-04-1 Prepared in cooperation with the State of Tennessee and with other agencies U.S. DEPARTMENT OF THE INTERIOR GALE A. NORTON, SECRETARY U.S. GEOLOGICAL SURVEY CHARLES G. GROAT, Director For information on the water program in Tennessee write to: District Chief, Water Resources Division U.S. Geological Survey 640 Grassmere Park, Suite 100 Nashville, Tennessee 37211 2005 PREFACE This volume of the annual hydrologic data report of Tennessee is one of a series of annual reports that document hydrologic data gathered from the U.S. Geological Survey’s surface- and ground-water data-collection networks in each State, Puerto Rico, and the Trust Territories. These records of streamflow, ground-water levels, and quality of water provide the hydrologic informa- tion needed by State, local, and Federal agencies, and the private sector for developing and man- aging our Nation’s land and water resources. This report is the culmination of a concerted effort by dedicated personnel of the U.S. Geo- logical Survey who collected, compiled, analyzed, verified, and organized the data, and who typed, edited, and assembled the report. In addition to the authors, who had primary responsibility for assuring that the information contained herein is accurate, complete, and adheres to Geologi- cal Survey policy and established guidelines, most of the data were collected, computed, and pro- cessed from the subdistrict offices. -
Tennessee Fish and Wildlife Commission Proclamation 18-13 Sport Fishing
Page 1 of 17 TENNESSEE FISH AND WILDLIFE COMMISSION PROCLAMATION 18-13 SPORT FISHING Pursuant to the authority granted by Title 70, Tennessee Code Annotated, Section 70-4-107, the Tennessee Fish and Wildlife Commission proclaims the following regulations effective March 1, 2019 . SECTION I. GENERAL SEASONS, AND STATEWIDE CREEL, POSSESSION, AND LENGTH LIMITS. A. GAME FISH SPECIES The season is open year-round on the following species, unless otherwise specified in this proclamation. The possession limit is twice the daily creel limit. Only the daily creel limit may be possessed while afield. It shall also be unlawful to possess while afield any fish, which has been altered to the extent that its species and/or total body length cannot be determined. The length of a fish shall be determined with the fish laying on a flat ruler, the mouth closed , and the caudal (tail) fin lobes squeezed so as to produce the maximum length. The mouth of the fish may not be manipulated or extended. Unless stated otherwise a slot limit is a protected length range within which no fish may be harvested. Statewide daily creel and length limits for fish species are listed in the table below which includes exceptions for specific waterbodies. Additional exceptions are listed in Sections Ill , IV, V, VI, and XIII. See Special Definitions (Section XIV) for reservoir boundary and specific area descriptions. Daily Length Limit Creel (minimum unless Species Limit otherwise stated) Rock bass and shadow bass (both species in combination) 20 None From Calderwood Reservoir -

Tennessee Fishing Regulations 2008
FISHING REGULATIONS Effective March 1, 2008 through February 28, 2009 2008 TENNESSEE ISHING F G U I D E FI S FREE JU HI N N E 7 , 2G 0 0 8 D A Y Tennessee Wildlife Resources Agency www.tnwildlife.org Funding Fisheries Programs • Tennessee’s Lake Sturgeon Project Bill Dance’s Three Key Words • Aquatic Nuisance Species Become a Millionaire with FLW FantasyFishing.com Bass Pro Shops® Outdoor World® is not only a spectacular outdoor store—it’s MUCH more! Our lodge-style architecture—complete with waterfalls, rustic wildlife displays, and huge aquariums stocked with trophy fish—offers a breath- taking backdrop to the area’s biggest selection of golf, fishing, hunting, camping, and boating gear. Browse through the expansive TRACKER® Marine Boat Center Indoor Showroom. So bring the entire family and plan on spending the day. It’ll be an outdoor adventure you’ll never forget! Memphis: I-40 at Sycamore View Road, Exit 12 6140 Macon Road Memphis, TN (901) 213-5800 Hours: Mon–Sat 9am–10pm, Sun 10am–7pm For an adventure in itself Nashville: 323 Opry Mills Drive Nashville, TN Shop online at For a FREE catalog (615) 514-5200 Boat: (615) 514-5180 Visit Our Stores Hours: Mon–Sat 9am–10pm, Sun 9am–7pm basspro.com 1-800-BASS PRO Sevierville: I-40, Exit 407 3629 Outdoor Nationwide Sportsman’s Place Kodak, TN (865) 932-5600 Hours: Mon–Sat 9am–10pm, Sun 9am–8pm BP321802 FEATURES 18 6 FUNDING FISHERIES PROGRAMS Have you ever wondered how your fishing license revenue is spent? Hopefully after reading this article you will understand not only where it is spent but also how and why it is spent as it is. -

TVA Hydroelectric System NRHP
NPS Form 10-900-b (Rev. 01/2009) OMB No. 1024-0018 (Expires 5/31/2015) United States Department of the Interior National Park Service National Register of Historic Places Multiple Property Documentation Form This form is used for documenting property groups relating to one or several historic contexts. See instructions in National Register Bulletin How to Complete the Multiple Property Documentation Form (formerly 168). Complete each item by entering the requested information. For additional space, use continuation sheets (Form 10-900-a). Use a typewriter, word processor, or computer to complete all items X New Submission Amended Submission A. Name of Multiple Property Listing Historic Resources of the Tennessee Valley Authority Hydroelectric System, 1933-1979 B. Associated Historic Contexts (Name each associated historic context, identifying theme, geographical area, and chronological period for each.) 1. The Character of the Tennessee Valley and the Coming of TVA 2. The Tennessee Valley Authority, 1933-1945 3. The Tennessee Valley Authority, 1945-1979 C. Form Prepared by name/title Andra Martens/Cathleen Collett/Phil Thomason/Rebecca Hightower date 11/11/2015 organization Thomason and Associates telephone ....:6=-1.:....:5=---=3..::.8..::.5_-4:....:9:...:6:...:0:.__ _____ street & number P.O. Box 121225 email [email protected] city or town Nashville state TN zip code 37212 ~~~~~----------------------------------- D. Certification Date of Action 1 NPS Form 10-900-b (Rev. 01/2009) OMB No. 1024-0018 (Expires 5/31/2015) Alabama, Georgia Historic Resources of the Tennessee Valley Authority Kentucky, North Hydroelectric System, 1933-1979 Carolina and Tennessee Name of Multiple Property Listing State Table of Contents for Written Narrative Provide the following information on continuation sheets. -

Indiana Coastal Nonpoint Source Pollution Control Plan
Indiana Coastal Nonpoint Pollution Control Program February 2005 This page was left blank intentionally Indiana Coastal Nonpoint Pollution Control Program Submitted To: National Atmospheric Oceanic Administration, Office of Coastal Resource Management U.S. Environmental Protection Agency Prepared By: Indiana Department of Natural Resources Lake Michigan Coastal Program In conjunction with the following partners Indiana Department of Environmental Management Indiana Department of Natural Resources, Division of Soil Conservation Purdue University, Agriculture Extension Special Thanks to: 6217 Workgroup Members Funding for this document made possible in part by the National Oceanic Atmospheric Administration, Office of Coastal Resource Management under authority of the Coastal Zone Management Act. This page was left blank intentionally Table of Contents Page Chapter 1 – Introduction 1 A -- Overview 1 B -- Nonpoint Source Pollution – Defined 3 C -- Partners in Nonpoint Source Pollution 4 D -- Boundary Area 4 Indiana’s Coastal Waters 5 Calumet River Basin 7 Diverted Chicago Watershed 9 St. Joseph River Basin – Lake Michigan Watershed 10 Maumee River Basin 12 Proposed Indiana Coastal Nonpoint Pollution Program Boundary 14 E -- Coastal Nonpoint Pollution Area Background Information 15 Land Use of the Region 15 Climate 18 Geology and Soils 18 Lakes 19 Wetlands 20 Rivers and Streams 21 Chapter 2 – Management Measures for Agriculture Sources 23 A – Introduction 23 B – Potential Sources of Agricultural Nonpoint Pollution in the CNPCP Watershed 27 Erosion from Cropland 27 Facility Wastewater & Runoff Control from Confined Animal Facilities 30 Application of Nutrients to Cropland 31 Application of Pesticides to Cropland 34 Grazing Management 36 Irrigation of Cropland (Justification for Exclusion) 37 C – Agricultural Management Measures to be Implemented 37 1.