Catalytic Processes from Biomass-Derived Hexoses and Pentoses: a Recent Literature Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterisation and Enzymic Degradation of Non-Starch Polysaccharides in Lignocellulosic By-Products
CHARACTERISATION AND ENZYMIC DEGRADATION OF NON-STARCH POLYSACCHARIDES IN LIGNOCELLULOSIC BY-PRODUCTS A study on sunflower meal and palm-kernel meal l( OC\ "i Promotoren: dr.ir. A.G.J. Voragen hoogleraar in de levensmiddelenchemie dr. W. Pilnik emeritus-hoogleraar in de levensmiddelenleer fifNOftZOl /S^3 E.-M. Dusterhoft CHARACTERISATION AND ENZYMIC DEGRADATION OF NON- STARCH POLYSACCHARIDES IN LIGNOCELLULOSIC BY PRODUCTS A study on sunflower meal and palm-kernel meal Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen op gezag van de rector magnificus, dr. H.C. van der Plas in het openbaar te verdedigen op woensdag 24 februari 1993 des namiddags te vier uur in de Aula van de Landbouwuniversiteit te Wageningen 0000 0512 8810 u>n SJLJ^Q CIP-DATA KONINKLUKEBIBLIOTHEEK , DEN HAAG Dusterhoft, Eva-Maria Characterisation and enzymic degradation of non-starch polysaccharides in lignocellulosic by-products: a study on sunflower meal and palm-kernel meal / Eva-Maria Dusterhoft. - [S.l.:s.n.] Thesis Wageningen.- With ref.- With summary in Dutch. ISBN 90-5485-076-0 Subject headings: non-starch polysaccharides / Helianthus annuus / Elaeis guineensis BlliLi .i :.;.;.:. LANDBOUWLNiVLRiHiol; ffiAGEMNGEN The research described in this thesis was financially supported by BP Nutrition Nederland B.V. and by a grant of the Dutch Ministry of Economic Affairs (subsidiary agreement 'Programmatische Bedrijfsgerichte Technologie Stimulering' (PBTS). fJNO^W! i 1-5^3 STELLINGEN 1) Kennis van alleen de suikersamenstelling van een heterogeen substraat is niet voldoende voor een voorspelling van de benodigde enzymen voor de hydrolyse ervan. dit proefschrift 2) De vertaling, die Thibault en Crepeau (1989) geven van de suikersamenstelling van zonnepitdoppen naar de daarin aanwezige polysacchariden, is gedeeltelijk onjuist. -

GRAS Notice 789 for Erythritol
GRAS Notice (GRN) No. 789 https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory. Toi• Strategies ~~~~G~~[)) JUN 7 20'8 Innovative solutions Sound science OFFICE OF FOOD ADDITIVE SAFE1Y June 5, 2018 Dr. Dennis Keefe Director, Division of Biotechnology and GRAS Notice Review Office of Food Additive Safety (HFS-200) Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway College Park, MD 20740-3835 Subject: GRAS Notification - Erythritol Dear Dr. Keefe: On behalf of Cargill, Incorporated, ToxStrategies, Inc. (its agent) is submitting, for FDA review, a copy of the GRAS notification as required. The enclosed document provides notice of a claim that the food ingredient, erythritol, described in the enclosed notification is exempt from the premarket approval requirement of the Federal Food, Drug, and Cosmetic Act because it has been determined to be generally recognized as safe (GRAS), based on scientific procedures, for addition to food. If you have any questions or require additional information, please do not hesitate to contact me at 630-352-0303, or [email protected]. Sincerely, (b) (6) Donald F. Schmitt, M.P.H. Senior Managing Scientist ToxStrategies, Inc., 931 W. 75th St. , Suite 137, PMB 263, Naperville, IL 60565 1 Office (630) 352-0303 • www.toxstrategies.com GRAS Determination of Erythritol for Use in Human Food JUNES,2018 Innovative solutions s ,..,.,',--.r-.r--.r--. OFFICE OF FOOD ADDITIVE SAFE1Y GRAS Determination of Erythritol for Use in Human Food SUBMITTED BY: Cargill, Incorporated 15407 McGinty Road West Wayzata, MN 55391 SUBMITTED TO: U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food Additive Safety HFS-200 5100 Paint Branch Parkway College Park MD 20740-3835 CONTACT FOR TECHNICAL OR OTIIER INFORMATION Donald F. -

Fermentation of Glucose and Xylose to Hydrogen in the Presence of Long Chain Fatty Acids
University of Windsor Scholarship at UWindsor Electronic Theses and Dissertations Theses, Dissertations, and Major Papers 2009 Fermentation of Glucose and Xylose to Hydrogen in the Presence of Long Chain Fatty Acids Stephen Reaume University of Windsor Follow this and additional works at: https://scholar.uwindsor.ca/etd Recommended Citation Reaume, Stephen, "Fermentation of Glucose and Xylose to Hydrogen in the Presence of Long Chain Fatty Acids" (2009). Electronic Theses and Dissertations. 92. https://scholar.uwindsor.ca/etd/92 This online database contains the full-text of PhD dissertations and Masters’ theses of University of Windsor students from 1954 forward. These documents are made available for personal study and research purposes only, in accordance with the Canadian Copyright Act and the Creative Commons license—CC BY-NC-ND (Attribution, Non-Commercial, No Derivative Works). Under this license, works must always be attributed to the copyright holder (original author), cannot be used for any commercial purposes, and may not be altered. Any other use would require the permission of the copyright holder. Students may inquire about withdrawing their dissertation and/or thesis from this database. For additional inquiries, please contact the repository administrator via email ([email protected]) or by telephone at 519-253-3000ext. 3208. Fermentation of Glucose and Xylose to Hydrogen in the Presence of Long Chain Fatty Acids by Stephen Reaume A Thesis Submitted to the Faculty of Graduate Studies through the Environmental Engineering Program in Partial Fulfillment of the Requirements for the Degree of Master of Applied Science at the University of Windsor Windsor, Ontario, Canada 2009 © 2009 Stephen Reaume AUTHORS DECLARATION OF ORIGINALITY I hereby certify that I am the sole author of this thesis and that no part of this thesis has been published or submitted for publication. -
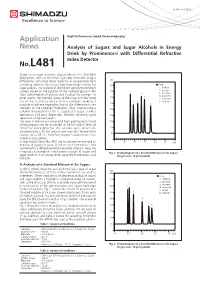
Analysis of Sugars and Sugar Alcohols in Energy Drink by Prominence-I with Differential Refreactive Index Detector
LAAN-A-LC-E258 Application High Performance Liquid Chromatography News Analysis of Sugars and Sugar Alcohols in Energy Drink by Prominence-i with Differential Refractive Index Detector No.L481 Sugars and sugar alcohols display almost no ultraviolet absorption, and are therefore typically detected using a differential refractive index detector or evaporative light uRI scattering detector. By using a ligand exchange column for 80 ■ Peaks sugar analysis, it is possible to distinguish among the different 1. maltose 70 2. glucose isomers based on the position of the hydroxyl group in the 1 4 3. fructose chair conformation of glucose and fructose for example. In 4. erythritol 60 5 other words, the hydroxyl group of the sugar and the metal 5. mannitol ion of the stationary phase form a complex, making it 2 3 6. sorbitol possible to achieve separation due to the difference in the 50 6 strength of the complex formation. Also, maintaining a 40 column temperature of 80 °C suppresses sugar anomer separation and peak dispersion, thereby achieving good 30 separation of adjacent peaks. The new Prominence-i integrated high-performance liquid 20 chromatograph can be connected to the RID-20A differential refractive index detector. The column oven, which can 10 accommodate a 30 cm column and maintain temperature 0 control up to 85 °C, therefore supports applications that require a long column. In Application News No. 467, we introduced an example of 0 5 10 15 20 25 min analysis of sugars in juice, in which the Prominence-i was connected to a differential refractive index detector. Here, we introduce an example of simultaneous analysis of sugars and Fig. -

Acute and Chronic Effects of Dietary Lactose in Adult Rats Are Not Explained by Residual Intestinal Lactase Activity
Nutrients 2015, 7, 5542-5555; doi:10.3390/nu7075237 OPEN ACCESS nutrients ISSN 2072-6643 www.mdpi.com/journal/nutrients Article Acute and Chronic Effects of Dietary Lactose in Adult Rats Are not Explained by Residual Intestinal Lactase Activity Bert J. M. van de Heijning *, Diane Kegler, Lidewij Schipper, Eline Voogd, Annemarie Oosting and Eline M. van der Beek Danone Nutricia Research, PO Box 80141, TC Utrecht 3508, The Netherlands; E-Mails: [email protected] (D.K.); [email protected] (L.S.); [email protected] (E.V.); [email protected] (A.O.); [email protected] (E.M.B.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +31-30-2095-000. Received: 5 June 2015 / Accepted: 30 June 2015 / Published: 8 July 2015 Abstract: Neonatal rats have a high intestinal lactase activity, which declines around weaning. Yet, the effects of lactose-containing products are often studied in adult animals. This report is on the residual, post-weaning lactase activity and on the short- and long-term effects of lactose exposure in adult rats. Acutely, the postprandial plasma response to increasing doses of lactose was studied, and chronically, the effects of a 30% lactose diet fed from postnatal (PN) Day 15 onwards were evaluated. Intestinal lactase activity, as assessed both in vivo and in vitro, was compared between both test methods and diet groups (lactose vs. control). A 50%–75% decreased digestive capability towards lactose was observed from weaning into adulthood. Instillation of lactose in adult rats showed disproportionally low increases in plasma glucose levels and did not elicit an insulin response. -

Xylose Fermentation to Ethanol by Schizosaccharomyces Pombe Clones with Xylose Isomerase Gene." Biotechnology Letters (8:4); Pp
NREL!TP-421-4944 • UC Category: 246 • DE93000067 l I Xylose Fermenta to Ethanol: A R ew '.) i I, -- , ) )I' J. D. McMillan I ' J.( .!i �/ .6' ....� .T u�.•ls:l ., �-- • National Renewable Energy Laboratory II 'J 1617 Cole Boulevard Golden, Colorado 80401-3393 A Division of Midwest Research Institute Operated for the U.S. Department of Energy under Contract No. DE-AC02-83CH10093 Prepared under task no. BF223732 January 1993 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, com pleteness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily con stitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. Printed in the United States of America Available from: National Technical Information Service U.S. Department of Commerce 5285 Port Royal Road Springfield, VA22161 Price: Microfiche A01 Printed Copy A03 Codes are used for pricing all publications. The code is determined by the number of pages in the publication. Information pertaining to the pricing codes can be found in the current issue of the following publications which are generally available in most libraries: Energy Research Abstracts (ERA); Govern ment Reports Announcements and Index ( GRA and I); Scientific and Technical Abstract Reports(STAR); and publication NTIS-PR-360 available from NTIS at the above address. -

Enhanced Trehalose Production Improves Growth of Escherichia Coli Under Osmotic Stress† J
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, July 2005, p. 3761–3769 Vol. 71, No. 7 0099-2240/05/$08.00ϩ0 doi:10.1128/AEM.71.7.3761–3769.2005 Copyright © 2005, American Society for Microbiology. All Rights Reserved. Enhanced Trehalose Production Improves Growth of Escherichia coli under Osmotic Stress† J. E. Purvis, L. P. Yomano, and L. O. Ingram* Department of Microbiology and Cell Science, Box 110700, University of Florida, Gainesville, Florida 32611 Downloaded from Received 7 July 2004/Accepted 9 January 2005 The biosynthesis of trehalose has been previously shown to serve as an important osmoprotectant and stress protectant in Escherichia coli. Our results indicate that overproduction of trehalose (integrated lacI-Ptac-otsBA) above the level produced by the native regulatory system can be used to increase the growth of E. coli in M9-2% glucose medium at 37°C to 41°C and to increase growth at 37°C in the presence of a variety of osmotic-stress agents (hexose sugars, inorganic salts, and pyruvate). Smaller improvements were noted with xylose and some fermentation products (ethanol and pyruvate). Based on these results, overproduction of trehalose may be a useful trait to include in biocatalysts engineered for commodity chemicals. http://aem.asm.org/ Bacteria have a remarkable capacity for adaptation to envi- and lignocellulose (6, 7, 10, 28, 30, 31, 32, 45). Biobased pro- ronmental stress (39). A part of this defense system involves duction of these renewable chemicals would be facilitated by the intracellular accumulation of protective compounds that improved growth under thermal stress and by increased toler- shield macromolecules and membranes from damage (9, 24). -

United States Patent Office
United States Patentaw Office ... ber of ether groupings on each molecule. These mix tures, however, if they analyze as containing an average 2,978,421. number of ether groups per molecule greater than one, NITRILE COPOLYMERS AND METHOD OF are capable of producing the insoluble nitrile polymers PREPARING SAME of this invention. Since the efficiency of the polyether cross-linking agent increases with the number of poten John A. Holloway, Cleveland, Ohio, assignor to The tially polymerizable groups on the molecule, it is much B. F. Goodrich Company, New York, N.Y., a corpo preferred to utilize polyethers containing an average of ration of New York - two or more alkenyl ether groupings per molecule. The No Drawing. Filed June 9, 1958, Ser. No. 740,566. 10 polyvinyl polyethers of the polyhydric alcohols within the above broad class are produced by reacting acetylene 9 Claims. (C. 260-17.4) with the polyhydric alcohol (or an alcoholate thereof) in a Reppe-type vinylation synthesis. The polycrotyl ethers of the polyhydric alcohols are also useful although they This invention relates to cross-linked alpha-beta ole 15 do not contain a terminal CHCC group. - finically unsaturated nitrile polymers and more particu Illustrative polyhydric alcohols of the above-described larly pertains to copolymers of alpha-beta olefinically class that may be utilized in the preparation of the poly. unsaturated nitriles and polyalkenyl polyethers of poly alkenyl polyether, cross-linking agent include the butane hydric alcohols and methods for their preparation. triols such as 1,2,3-butane triol, 2,3,4-trihydroxybutyric An object of this invention is the provision of insoluble, 20 acid, the aldotetroses such as erythrose and threose, keto cross-linked polynitriles which are capable of thickening tetroses such as erythrulose; the aldopentoses such as certain non-aqueous polar solvents. -

Structure-Function Relationships of Glucansucrase and Fructansucrase Enzymes from Lactic Acid Bacteria Sacha A
MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Mar. 2006, p. 157–176 Vol. 70, No. 1 1092-2172/06/$08.00ϩ0 doi:10.1128/MMBR.70.1.157–176.2006 Copyright © 2006, American Society for Microbiology. All Rights Reserved. Structure-Function Relationships of Glucansucrase and Fructansucrase Enzymes from Lactic Acid Bacteria Sacha A. F. T. van Hijum,1,2†* Slavko Kralj,1,2† Lukasz K. Ozimek,1,2 Lubbert Dijkhuizen,1,2 and Ineke G. H. van Geel-Schutten1,3 Centre for Carbohydrate Bioprocessing, TNO-University of Groningen,1 and Department of Microbiology, Groningen Biomolecular Sciences and Biotechnology Institute, University of Groningen,2 9750 AA Haren, The Netherlands, and Innovative Ingredients and Products Department, TNO Quality of Life, Utrechtseweg 48 3704 HE Zeist, The Netherlands3 INTRODUCTION .......................................................................................................................................................157 NOMENCLATURE AND CLASSIFICATION OF SUCRASE ENZYMES ........................................................158 GLUCANSUCRASES .................................................................................................................................................158 Reactions Catalyzed and Glucan Product Synthesis .........................................................................................161 Glucan synthesis .................................................................................................................................................161 Acceptor reaction ................................................................................................................................................161 -

Mannoside Recognition and Degradation by Bacteria Simon Ladeveze, Elisabeth Laville, Jordane Despres, Pascale Mosoni, Gabrielle Veronese
Mannoside recognition and degradation by bacteria Simon Ladeveze, Elisabeth Laville, Jordane Despres, Pascale Mosoni, Gabrielle Veronese To cite this version: Simon Ladeveze, Elisabeth Laville, Jordane Despres, Pascale Mosoni, Gabrielle Veronese. Mannoside recognition and degradation by bacteria. Biological Reviews, Wiley, 2016, 10.1111/brv.12316. hal- 01602393 HAL Id: hal-01602393 https://hal.archives-ouvertes.fr/hal-01602393 Submitted on 26 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Biol. Rev. (2016), pp. 000–000. 1 doi: 10.1111/brv.12316 Mannoside recognition and degradation by bacteria Simon Ladeveze` 1, Elisabeth Laville1, Jordane Despres2, Pascale Mosoni2 and Gabrielle Potocki-Veron´ ese` 1∗ 1LISBP, Universit´e de Toulouse, CNRS, INRA, INSA, 31077, Toulouse, France 2INRA, UR454 Microbiologie, F-63122, Saint-Gen`es Champanelle, France ABSTRACT Mannosides constitute a vast group of glycans widely distributed in nature. Produced by almost all organisms, these carbohydrates are involved in numerous cellular processes, such as cell structuration, protein maturation and signalling, mediation of protein–protein interactions and cell recognition. The ubiquitous presence of mannosides in the environment means they are a reliable source of carbon and energy for bacteria, which have developed complex strategies to harvest them. -

Brazilian Potential for Biomass Ethanol: Challenge of Using Hexose and Pentose Co- Fermenting Yeast Strains
Journal of Scientific & Industrial Research 918Vol. 67, November 2008, pp.918-926 J SCI IND RES VOL 67 NOVEMBER 2008 Brazilian potential for biomass ethanol: Challenge of using hexose and pentose co- fermenting yeast strains Boris U Stambuk1*, Elis C A. Eleutherio2, Luz Marina Florez-Pardo3 Ana Maria Souto-Maior4 and Elba P S Bon5 1Laboratório de Biologia Molecular e Biotecnologia de Leveduras, Centro de Ciências Biológicas, Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil 2Laboratório de Investigação de Fatores de Estresse, Instituto de Química, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil 3Departamento Sistemas de Produccion, Universidad Autonoma de Occidente, Cali, Colombia 4Departamento de Antibióticos, Universidade Federal de Pernambuco, Recife, PE, Brasil 5Laboratório de Tecnologia Enzimática, Instituto de Química, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil Received 15 July 2008; revised 16 September 2008; accepted 01 October 2008 This paper reviews Brazilian scenario and efforts for deployment of technology to produce bioethanol vis-à-vis recent international advances in the area, including possible use of hexose and pentose co-fermenting yeast strains. Keywords: Biomass ethanol, Brazilian ethanol production, Ethanologenic yeasts, Hexose-pentose co-fermentation Introduction ethanol, 109 produce only ethanol and 16 produce only Brazil produces annually 5 x 109 gallons of ethanol sugar. Brazil will produce around 27 billion litre of ethanol from sugarcane grown on 5 million ha. Sugarcane is in 2008, and plans to build 41 new distilleries before 2010, processed in 365 sugar/ethanol producing units, and it and 45 more until 20156. Land use for energy crops as is forecasted that 86 new distilleries will be built before compared to food crops has been opted for renewable 2015. -

406-3 Wood Sugars.Pdf
Wood Chemistry Wood Chemistry Wood Carbohydrates l Major Components Wood Chemistry » Hexoses – D-Glucose, D-Galactose, D-Mannose PSE 406/Chem E 470 » Pentoses – D-Xylose, L-Arabinose Lecture 3 » Uronic Acids Wood Sugars – D-glucuronic Acid, D Galacturonic Acid l Minor Components » 2 Deoxy Sugars – L-Rhamnose, L-Fucose PSE 406 - Lecture 3 1 PSE 406 - Lecture 3 2 Wood Chemistry Wood Sugars: L Arabinose Wood Chemistry Wood Sugars: D Xylose l Pentose (5 carbons) CHO l Pentose CHO l Of the big 5 wood sugars, l Xylose is the major constituent of H OH arabinose is the only one xylans (a class of hemicelluloses). H OH found in the L form. » 3-8% of softwoods HO H HO H l Arabinose is a minor wood » 15-25% of hardwoods sugar (0.5-1.5% of wood). HO H H OH CH OH 2 CH2OH PSE 406 - Lecture 3 3 PSE 406 - Lecture 3 4 1 1 Wood Chemistry Wood Sugars: D Mannose Wood Chemistry Wood Sugars: D Glucose CHO l Hexose (6 carbons) CHO l Hexose (6 carbons) l Glucose is the by far the most H OH l Mannose is the major HO H constituent of Mannans (a abundant wood monosaccharide (cellulose). A small amount can HO H class of hemicelluloses). HO H also be found in the » 7-13% of softwoods hemicelluloses (glucomannans) H OH » 1-4% of hardwoods H OH H OH H OH CH2OH CH2OH PSE 406 - Lecture 3 5 PSE 406 - Lecture 3 6 Wood Chemistry Wood Sugars: D Galactose Wood Chemistry Wood Sugars CHO CHO l Hexose (6 carbons) CHO H OH H OH l Galactose is a minor wood D Xylose L Arabinose HO H HO H monosaccharide found in H OH HO H H OH certain hemicelluloses CH2OH HO H CHO CH2OH CHO CHO » 1-6% of softwoods HO H H OH H OH » 1-1.5% of hardwoods HO H HO H HO H HO H HO H H OH H OH H OH H OH H OH H OH CH2OH CH2OH CH2OH CH2OH D Mannose D Glucose D Galactose PSE 406 - Lecture 3 7 PSE 406 - Lecture 3 8 2 2 Wood Chemistry Sugar Numbering System Wood Chemistry Uronic Acids CHO 1 CHO l Aldoses are numbered l Uronic acids are with the structure drawn HO H 2 polyhydroxy carboxylic H OH vertically starting from the aldehydes.