AVMA Guidelines for the Euthanasia of Animals: 2013 Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enacted and Vetoed State Laws Affecting Animals for the Year 2003
ENACTED AND VETOED STATE LAWS AFFECTING ANIMALS FOR THE YEAR 2003 The following list is a compilation of laws and resolutions that were passed by state legislatures in 2003 and then signed into law or vetoed. The list includes legislation that had a positive, negative, or neutral impact on animals. Please e-mail Julie Janovsky at [email protected] , to obtain a copy of these bills, or if you have any questions, additions or corrections to the list. ALABAMA H.B. 37 (Buskey) - Greyhound Euthanasia Requires that the only method allowed for euthanasia of greyhounds is by lethal injection and makes it a crime to remove greyhounds from the state for the purpose of euthanizing. Signed into law on 6/20/03. ALASKA H.B. 208 (Fate) & S.B. 155 (Seekins) - Airborne Shooting Allows the Department of Game and Fish to authorize a predator control program involving airborne or same day airborne shooting of wolves. Signed into law on 6/18/03 S.B. 147 (Green) - NWCO BILL Provides for licensing and regulating of nuisance wildlife control operators. Signed into law on 6/28/03. ARIZONA H.B. 2121 (Carruthers) - Dogs Changes the minimum age for a dog to be considered a stray from four months to three months, and adds an additional $10 to the $2 late payment fee for a dog license if more than a year late, or an additional $20 if more than 2 years late. Signed into law on 5/6/03. S.B. 1351 (Weiers) - Pet Trusts Creates trusts for pets. Signed into law on 5/12/03. -

Management of Chronic Problems
MANAGEMENT OF CHRONIC PROBLEMS INTERACTIONS BETWEEN ALCOHOL AND DRUGS A. Leary,* T. MacDonald† SUMMARY concerned. Alcohol may alter the effects of the drug; drug In western society alcohol consumption is common as is may change the effects of alcohol; or both may occur. the use of therapeutic drugs. It is not surprising therefore The interaction between alcohol and drug may be that concomitant use of these should occur frequently. The pharmacokinetic, with altered absorption, metabolism or consequences of this combination vary with the dose of elimination of the drug, alcohol or both.2 Alcohol may drug, the amount of alcohol taken, the mode of affect drug pharmacokinetics by altering gastric emptying administration and the pharmacological effects of the drug or liver metabolism. Drugs may affect alcohol kinetics by concerned. Interactions may be pharmacokinetic or altering gastric emptying or inhibiting gastric alcohol pharmacodynamic, and while coincidental use of alcohol dehydrogenase (ADH).3 This may lead to altered tissue may affect the metabolism or action of a drug, a drug may concentrations of one or both agents, with resultant toxicity. equally affect the metabolism or action of alcohol. Alcohol- The results of concomitant use may also be principally drug interactions may differ with acute and chronic alcohol pharmacodynamic, with combined alcohol and drug effects ingestion, particularly where toxicity is due to a metabolite occurring at the receptor level without important changes rather than the parent drug. There is both inter- and intra- in plasma concentration of either. Some interactions have individual variation in the response to concomitant drug both kinetic and dynamic components and, where this is and alcohol use. -

AVMA Guidelines for the Euthanasia of Animals: 2020 Edition*
AVMA Guidelines for the Euthanasia of Animals: 2020 Edition* Members of the Panel on Euthanasia Steven Leary, DVM, DACLAM (Chair); Fidelis Pharmaceuticals, High Ridge, Missouri Wendy Underwood, DVM (Vice Chair); Indianapolis, Indiana Raymond Anthony, PhD (Ethicist); University of Alaska Anchorage, Anchorage, Alaska Samuel Cartner, DVM, MPH, PhD, DACLAM (Lead, Laboratory Animals Working Group); University of Alabama at Birmingham, Birmingham, Alabama Temple Grandin, PhD (Lead, Physical Methods Working Group); Colorado State University, Fort Collins, Colorado Cheryl Greenacre, DVM, DABVP (Lead, Avian Working Group); University of Tennessee, Knoxville, Tennessee Sharon Gwaltney-Brant, DVM, PhD, DABVT, DABT (Lead, Noninhaled Agents Working Group); Veterinary Information Network, Mahomet, Illinois Mary Ann McCrackin, DVM, PhD, DACVS, DACLAM (Lead, Companion Animals Working Group); University of Georgia, Athens, Georgia Robert Meyer, DVM, DACVAA (Lead, Inhaled Agents Working Group); Mississippi State University, Mississippi State, Mississippi David Miller, DVM, PhD, DACZM, DACAW (Lead, Reptiles, Zoo and Wildlife Working Group); Loveland, Colorado Jan Shearer, DVM, MS, DACAW (Lead, Animals Farmed for Food and Fiber Working Group); Iowa State University, Ames, Iowa Tracy Turner, DVM, MS, DACVS, DACVSMR (Lead, Equine Working Group); Turner Equine Sports Medicine and Surgery, Stillwater, Minnesota Roy Yanong, VMD (Lead, Aquatics Working Group); University of Florida, Ruskin, Florida AVMA Staff Consultants Cia L. Johnson, DVM, MS, MSc; Director, -

Euthanasia of Experimental Animals
EUTHANASIA OF EXPERIMENTAL ANIMALS • *• • • • • • • *•* EUROPEAN 1COMMISSIO N This document has been prepared for use within the Commission. It does not necessarily represent the Commission's official position. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server (http://europa.eu.int) Cataloguing data can be found at the end of this publication Luxembourg: Office for Official Publications of the European Communities, 1997 ISBN 92-827-9694-9 © European Communities, 1997 Reproduction is authorized, except for commercial purposes, provided the source is acknowledged Printed in Belgium European Commission EUTHANASIA OF EXPERIMENTAL ANIMALS Document EUTHANASIA OF EXPERIMENTAL ANIMALS Report prepared for the European Commission by Mrs Bryony Close Dr Keith Banister Dr Vera Baumans Dr Eva-Maria Bernoth Dr Niall Bromage Dr John Bunyan Professor Dr Wolff Erhardt Professor Paul Flecknell Dr Neville Gregory Professor Dr Hansjoachim Hackbarth Professor David Morton Mr Clifford Warwick EUTHANASIA OF EXPERIMENTAL ANIMALS CONTENTS Page Preface 1 Acknowledgements 2 1. Introduction 3 1.1 Objectives of euthanasia 3 1.2 Definition of terms 3 1.3 Signs of pain and distress 4 1.4 Recognition and confirmation of death 5 1.5 Personnel and training 5 1.6 Handling and restraint 6 1.7 Equipment 6 1.8 Carcass and waste disposal 6 2. General comments on methods of euthanasia 7 2.1 Acceptable methods of euthanasia 7 2.2 Methods acceptable for unconscious animals 15 2.3 Methods that are not acceptable for euthanasia 16 3. Methods of euthanasia for each species group 21 3.1 Fish 21 3.2 Amphibians 27 3.3 Reptiles 31 3.4 Birds 35 3.5 Rodents 41 3.6 Rabbits 47 3.7 Carnivores - dogs, cats, ferrets 53 3.8 Large mammals - pigs, sheep, goats, cattle, horses 57 3.9 Non-human primates 61 3.10 Other animals not commonly used for experiments 62 4. -

Pharmacology – Inhalant Anesthetics
Pharmacology- Inhalant Anesthetics Lyon Lee DVM PhD DACVA Introduction • Maintenance of general anesthesia is primarily carried out using inhalation anesthetics, although intravenous anesthetics may be used for short procedures. • Inhalation anesthetics provide quicker changes of anesthetic depth than injectable anesthetics, and reversal of central nervous depression is more readily achieved, explaining for its popularity in prolonged anesthesia (less risk of overdosing, less accumulation and quicker recovery) (see table 1) Table 1. Comparison of inhalant and injectable anesthetics Inhalant Technique Injectable Technique Expensive Equipment Cheap (needles, syringes) Patent Airway and high O2 Not necessarily Better control of anesthetic depth Once given, suffer the consequences Ease of elimination (ventilation) Only through metabolism & Excretion Pollution No • Commonly administered inhalant anesthetics include volatile liquids such as isoflurane, halothane, sevoflurane and desflurane, and inorganic gas, nitrous oxide (N2O). Except N2O, these volatile anesthetics are chemically ‘halogenated hydrocarbons’ and all are closely related. • Physical characteristics of volatile anesthetics govern their clinical effects and practicality associated with their use. Table 2. Physical characteristics of some volatile anesthetic agents. (MAC is for man) Name partition coefficient. boiling point MAC % blood /gas oil/gas (deg=C) Nitrous oxide 0.47 1.4 -89 105 Cyclopropane 0.55 11.5 -34 9.2 Halothane 2.4 220 50.2 0.75 Methoxyflurane 11.0 950 104.7 0.2 Enflurane 1.9 98 56.5 1.68 Isoflurane 1.4 97 48.5 1.15 Sevoflurane 0.6 53 58.5 2.5 Desflurane 0.42 18.7 25 5.72 Diethyl ether 12 65 34.6 1.92 Chloroform 8 400 61.2 0.77 Trichloroethylene 9 714 86.7 0.23 • The volatile anesthetics are administered as vapors after their evaporization in devices known as vaporizers. -

Advice on Euthanasia Techniques for Small and Large Cetaceans
Canadian Science Advisory Secretariat (CSAS) Research Document 2014/111 National Capital Region Advice on Euthanasia Techniques for Small and Large Cetaceans Pierre-Yves Daoust1 and Arthur Ortenburger2 1 Canadian Wildlife Health Cooperative Department of Pathology & Microbiology Atlantic Veterinary College University of Prince Edward Island 550 University Avenue Charlottetown, PE C1A 4P3 2 Department of Health Management Atlantic Veterinary College University of Prince Edward Island 550 University Avenue Charlottetown, PE C1A 4P3 January 2015 Foreword This series documents the scientific basis for the evaluation of aquatic resources and ecosystems in Canada. As such, it addresses the issues of the day in the time frames required and the documents it contains are not intended as definitive statements on the subjects addressed but rather as progress reports on ongoing investigations. Research documents are produced in the official language in which they are provided to the Secretariat. Published by: Fisheries and Oceans Canada Canadian Science Advisory Secretariat 200 Kent Street Ottawa ON K1A 0E6 http://www.dfo-mpo.gc.ca/csas-sccs/ [email protected] © Her Majesty the Queen in Right of Canada, 2015 ISSN 1919-5044 Correct citation for this publication: Daoust, P.-Y., Ortenburger, A. 2015. Advice on Euthanasia Techniques for Small and Large Cetaceans. DFO Can. Sci. Advis. Sec. Res. Doc. 2014/111. v + 36 p. TABLE OF CONTENTS I. ABSTRACT ....................................................................................................................... -

1999 Enacted and Vetoed Animal Legislation
1999 Enacted And Vetoed Animal Legislation The following list is a compilation of laws and resolutions that were passed by state legislatures in 1999 and then signed into law or vetoed. The list includes legislation that had a positive, negative, or neutral impact on animals. For a copy of any of these laws and resolutions, or if you have questions, additions, or corrections, please e-mail Julie Janovsky at [email protected]. ALASKA AK S.B. 74 Game Management Authorizes an employee of the Department of Fish and Game, as part of the game management program, to shoot or assist in shooting wolf, wolverine, fox, or lynx on the same day that the employee is airborne. Vetoed: July 9, 1999 ARIZONA AZ S.B. 1174 Animal Cruelty Penalties Establishes that intentionally, knowingly, or recklessly failing to provide medical attention necessary to prevent protracted suffering to any animal under a person's custody or control is a Class 1 misdemeanor; and establishes that subjecting an animal under a person's custody or control to cruel neglect or abandonment—which results in serious physical injury to the animal—is a Class 6 felony. Signed into law: May 4, 1999 AZ H.B. 2578 Specialty License Plates Allows an organization with less than 200 members to request approval for a special license plate, provided the organization agrees to pay the production and program costs of the special plate. Signed into law: May 4, 1999 ARKANSAS AR H.B. 1442 Animal Shelter Procedures Requires mandatory sterilization of dogs and cats released by pounds, shelters, and humane organizations; and applies to counties with a population of 300,000 or more. -

Diluted Isoflurane As a Suitable Alternative for Diethyl Ether for Rat Anaesthesia in Regular Toxicology Studies
FULL PAPER Laboratory Aminal Science Diluted Isoflurane as a Suitable Alternative for Diethyl ether for Rat Anaesthesia in Regular Toxicology Studies Toshiaki NAGATE1)*, Tomonobu CHINO1), Chizuru NISHIYAMA1), Daisuke OKUHARA1), Toru TAHARA1), Yoshimasa MARUYAMA1), Hiroko KASAHARA1), Kayoko TAKASHIMA1), Sayaka KOBAYASHI1), Yoshiyuki MOTOKAWA1), Shin-ichi MUTO1) and Junji KURODA1) 1)Toxicology Research Laboratory, R&D, Kissei Pharmaceutical Co., Ltd., 2320–1 Maki, Hotaka, Azumino-City, Nagano-Pref. 399–8305, Japan (Received 6 October 2006/Accepted 20 July 2007) ABSTRACT. Despite its explosive properties and toxicity to both animals and humans, diethyl ether is an agent long used in Japan in the anaesthesia jar method of rat anaesthetises. However, in response to a recent report from the Science Council of Japan condemning diethyl ether as acceptable practice, we searched for an alternative rat anaesthesia method that provided data continuous with pre-existing regular toxicology studies already conducted under diethyl ether anaesthesia. For this, we examined two candidates; 30% isoflurane diluted with propylene glycol and pentobarbitone. Whereas isoflurane is considered to be one of the representatives of modern volatile anaesthetics, the method of propylene glycol-diluted 30% isoflurane used in this study was our modification of a recently reported method revealed to have several advantages as an inhalation anaesthesia. Intraperitoneal pentobarbitone has long been accepted as a humane method in laboratory animal anaesthesiology. These 2 modalities were scrutinized in terms of consistency of haematology and blood chemistry with previous results using ether. We found that pentobarbitone required a much longer induction time than diethyl ether, which is suspected to be the cause of fluctuations in several haematological and blood chemical results. -

Full List of Lead Terms and Cross References
BIOETHICS THESAURUS 2011 ABORTED FETUSES ALTERNATIVE THERAPIES ABORTIFACIENTS ALTERNATIVES ABORTION ALTRUISM ABORTION ON DEMAND ALZHEIMER DISEASE Abortion, Induced Use ABORTION AMBULATORY CARE Abortion, Spontaneous Use MISCARRIAGE AMERICAN INDIANS ACADEMIC MEDICAL CENTERS AMNIOCENTESIS Access to Health Care Use HEALTH CARE AMYOTROPHIC LATERAL SCLEROSIS DELIVERY ANALOGY ACCESS TO INFORMATION ANCIENT HISTORY ACCOUNTABILITY ANENCEPHALY Acquired Immunodeficiency Syndrome Use AIDS ANESTHESIA ACTIVE EUTHANASIA Aneuploidy Use CHROMOSOME ADDICTION ABNORMALITIES ADMINISTRATORS ANIMAL BEHAVIOR ADOLESCENTS ANIMAL CARE COMMITTEES ADOPTION ANIMAL CLONING ADULT CHILDREN ANIMAL EUTHANASIA ADULT STEM CELLS ANIMAL EXPERIMENTATION ADULTERY ANIMAL GROUPS ADULTS ANIMAL ORGANS Adult-Onset Genetic Disorders Use LATE-ONSET ANIMAL PRODUCTION DISORDERS ANIMAL RIGHTS ADVANCE CARE PLANNING ANIMAL TESTING ALTERNATIVES Advance Directive Adherence Use ADVANCE Animal Use Alternatives Use ANIMAL TESTING DIRECTIVES and DIRECTIVE ALTERNATIVES ADHERENCE ANIMAL WELFARE ADVANCE DIRECTIVES Animals, Genetically Modified Use ADVERSE EFFECTS GENETICALLY MODIFIED ANIMALS ADVERTISING Animals, Transgenic Use GENETICALLY ADVISORY COMMITTEES MODIFIED ANIMALS AFRICAN AMERICANS ANONYMOUS TESTING AGE FACTORS Antenatal Diagnosis Use PRENATAL DIAGNOSIS AGED Antenatal Injuries Use PRENATAL INJURIES AGGRESSION ANTHROPOLOGY AGING APO-E GENES AGRICULTURE APTITUDE Ahliyah Use COMPETENCE ARAB WORLD AIDS ARABS AIDS SERODIAGNOSIS ARTIFICIAL FEEDING ALCOHOL ABUSE ARTIFICIAL INSEMINATION ALLIED -
Euthanasia Chart
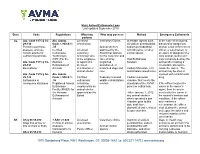
State Animal Euthanasia Laws Last updated September 2019 State Code Regulations Who may Who may possess Method Emergency Euthanasia perform AL Ala. Code 1975 § 34- Ala. Admin. Licensed Veterinary Clinics Injectable agents such In the case of an injured, 29-130 Code r. 930-X-1- veterinarian as sodium pentobarbital, diseased or dangerous Permit to purchase, .35 Animal shelters sodium pentobarbital animal, a law enforcement possess, and use Certified Licensed approved by the with lidocaine, or other officer, a veterinarian, or certain agents for Euthanasia veterinary Board that operate similar agents an agent or designee of a euthanizing animals Technicians technician who for the collection and local animal control unit (CET) For the is the employee care of stray, Oral Euthanasia may humanely destroy the Ala. Code 1975 § 34- Humane or agent of a neglected, Solution animal after making a 29-131 Euthanasia of licensed abandoned, or reasonable attempt to Exemptions Animals veterinarian or unwanted dogs and Carbon Monoxide, CO, locate the owner. The animal shelter cats and inhalant anesthetics animal may be shot or Ala. Code 1975 § 34- Ala. Admin. injected with a barbiturate 29-132 Code r. 930-X-1- Certified Federally licensed Carbon monoxide drug. Euthanasia in .36 euthanasia wildlife rehabilitation chamber that meets the emergency situations Registered Animal technician centers standards of the AVMA If the officer locates the Euthanasia employed by an panel on euthanasia owner or the owner’s Facility (RAEF) for animal shelter agent, then he or she the Humane approved by the *After January 1, 2012, must notify the owner of Euthanasia of Board any animal shelters the animal’s location and Animals which operated a gas condition. -

Pharmacokinetics of Ovarian Steroids in Sprague-Dawley Rats After Acute Exposure to 2,3,7,8-Tetrachlorodibenzo- P-Dioxin (TCDD)
Vol. 3, No. 2 131 ORIGINAL PAPER Pharmacokinetics of ovarian steroids in Sprague-Dawley rats after acute exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) Brian K. Petroff 1,2,3 and Kemmy M. Mizinga4 2Department of Molecular and Integrative Physiology,Physiology, 3Center for Reproductive Sciences, University of Kansas Medical Center, Kansas City, KS 66160. 4Department of Pharmacology,Pharmacology, University of Health Sciences, Kansas City,City, MO 64106 Received: 3 June 2003; accepted: 28 June 2003 SUMMARY 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces abnormalities in ste- roid-dependent processes such as mammary cell proliferation, gonadotropin release and maintenance of pregnancy. In the current study, the effects of TCDD on the pharmacokinetics of 17ß-estradiol and progesterone were examined. Adult Sprague-Dawley rats were ovariectomized and pretreated with TCDD (15 µg/kg p.o.) or vehicle. A single bolus of 17ß-estradiol (E2, 0.3 µmol/kg i.v.) or progesterone (P4, 6 µmol/kg i.v.) was administered 24 hours after TCDD and blood was collected serially from 0-72 hours post- injection. Intravenous E2 and P4 in DMSO vehicle had elimination half-lives of approximately 10 and 11 hours, respectively. TCDD had no signifi cant effect on the pharmacokinetic parameters of P4. The elimination constant 1Corresponding author: Center for Reproductive Sciences, Department of Molecular and Integra- tive Physiology, University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66160, USA; e-mail: [email protected] Copyright © 2003 by the Society for Biology of Reproduction 132 TCDD and ovarian steroid pharmacokinetics and clearance of E2 were decreased by TCDD while the elimination half-life, volume of distribution and area under the time*concentration curve were not altered signifi cantly. -

Objective Salicylate Propionic Acid Derivatives Acetic Acid
Semester-IV Sub Name-medicinal chemistry-I (sub code-BP-402T) Objective Sodium salicylate, Aspirin, Mefenamic acid*, Meclofenamate, Indomethacin, Sulindac, Tolmetin, Zomepriac, Diclofenac, Ketorolac, Ibuprofen*, Naproxen, Piroxicam, Phenacetin, Acetaminophen, Antipyrine, Phenylbutazone. 1. INTRODUCTION A drug or substance that reduces inflammation (redness, swelling, and pain) in the body. Anti- inflammatory agents block certain substances in the body that cause inflammation. They are used to treat many different conditions. Some anti-inflammatory agents are being studied in the prevention and treatment of cancer. 1.1 CLASSIFICATION NSAIDs can be classified based on their chemical structure or mechanism of action. Older NSAIDs were known long before their mechanism of action was elucidated and were for this reason classified by chemical structure or origin. Newer substances are more often classified by mechanism of action. Salicylate • Aspirin (acetylsalicylic acid) • Diflunisal (Dolobid) • Salicylic acid and its salts Propionic acid derivatives • Ibuprofen • Dexibuprofen • Naproxen • Fenoprofen • Ketoprofen • Dexketoprofen Acetic acid derivatives • Indomethacin • Tolmetin • Sulindac • Ketorolac • Diclofenac • Aceclofenac • Nabumetone (drug itself is non-acidic but the active, principal metabolite has a carboxylic acid group) Enolic acid (oxicam) derivatives • Piroxicam • Meloxicam • Tenoxicam • Droxicam • Lornoxicam • Isoxicam (withdrawn from market 1985) • Phenylbutazone Anthranilic acid derivatives (fenamates) The following NSAIDs are