Chem 112 – Experiment 5 – Simulation – Ph Indicators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Fiber Optic Ammonia Sensor Using a Universal Ph Indicator
Sensors 2014, 14, 4060-4073; doi:10.3390/s140304060 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Article A Fiber Optic Ammonia Sensor Using a Universal pH Indicator Adolfo J. Rodríguez 1,*, Carlos R. Zamarreño 2, Ignacio R. Matías 2, Francisco. J. Arregui 2, Rene F. Domínguez Cruz 1 and Daniel. A. May-Arrioja 1 1 Fiber and Integrated Optics Laboratory, Electronics Engineering Department, UAM Reynosa Rodhe, Universidad Autónoma de Tamaulipas, Carr. Reynosa-San Fernando S/N, Reynosa, Tamaulipas 88779, Mexico; E-Mails: [email protected] (R.F.D.C.); [email protected] (D.A.M.-A.) 2 Departamento de Ingeniería Eléctrica y Electrónica, Universidad Pública de Navarra, Edif. Los Tejos, Campus Arrosadia, Pamplona España 31006, Spain; E-Mails: [email protected] (C.R.Z.); [email protected] (I.R.M.); [email protected] (F.J.A.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +52-899-9213300 (ext. 8315); Fax: +52-899-921-3300 (ext. 8050). Received: 27 December 2013; in revised form: 12 February 2014 / Accepted: 18 February 2014 / Published: 27 February 2014 Abstract: A universal pH indicator is used to fabricate a fiber optic ammonia sensor. The advantage of this pH indicator is that it exhibits sensitivity to ammonia over a broad wavelength range. This provides a differential response, with a valley around 500 nm and a peak around 650 nm, which allows us to perform ratiometric measurements. The ratiometric measurements provide not only an enhanced signal, but can also eliminate any external disturbance due to humidity or temperature fluctuations. -

Detergent Residue Testing Using a Ph Meter, Ph Indicator, Or Test Kit
Alconox, Inc. The Leader in Critical Cleaning 30 Glenn St. White Plains NY 10603 USA Tel 914.948.4040 Fax 914-948-4088 www.alconox.com [email protected] Detergent Residue Testing Using a pH Meter, pH indicator, or Test Kit The following test procedures are suitable for detecting detergent residues resulting from improper rinsing and can be used to meet laboratory accreditation guidelines and questionnaires such as the College of American Pathologist program of State water lab accreditation programs. A. pH Meter Method 1. Rinse a small clean beaker by filling and emptying 3 times with source water. 2. Fill a 4th time and measure pH using a pH meter. Record the pH as source water pH. 3. Using a piece of cleaned glassware you wish to test, fill about 10% full with source water (10ml into 100ml beaker). Use more water if necessary to get enough water to be able to sufficiently immerse the pH meter electrode in your measuring beaker. 4. Swish water in glassware to extract residues from all possible surfaces. 5. Take pH reading with pH meter and record as glassware pH. 6. Any significant increase in pH indicates possible alkaline detergent residue. A significant change is 0.2 or more pH units on a pH meter measuring to 0.1 pH units of sensitivity. A result of less than 0.2-pH units change indicates properly rinsed glassware. Note: If deionized water is used as the sample water, a slight amount (10-20 mg/L) of reagent grade, non-buffering salt (NaCl, CaCl2) should be added to the sample water to allow pH meter to function properly. -
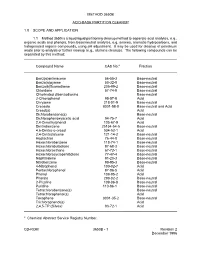
Method 3650B: Acid-Base Partition Cleanup, Part of Test Methods For
METHOD 3650B ACID-BASE PARTITION CLEANUP 1.0 SCOPE AND APPLICATION 1.1 Method 3650 is a liquid-liquid partitioning cleanup method to separate acid analytes, e.g., organic acids and phenols, from base/neutral analytes, e.g. amines, aromatic hydrocarbons, and halogenated organic compounds, using pH adjustment. It may be used for cleanup of petroleum waste prior to analysis or further cleanup (e.g., alumina cleanup). The following compounds can be separated by this method: _______________________________________________________________________________ Compound Name CAS No.a Fraction Benz(a)anthracene 56-55-3 Base-neutral Benzo(a)pyrene 50-32-8 Base-neutral Benzo(b)fluoranthene 205-99-2 Base-neutral Chlordane 57-74-9 Base-neutral Chlorinated dibenzodioxins Base-neutral 2-Chlorophenol 95-57-8 Acid Chrysene 218-01-9 Base-neutral Creosote 8001-58-9 Base-neutral and Acid Cresol(s) Acid Dichlorobenzene(s) Base-neutral Dichlorophenoxyacetic acid 94-75-7 Acid 2,4-Dimethylphenol 105-67-9 Acid Dinitrobenzene 25154-54-5 Base-neutral 4,6-Dinitro-o-cresol 534-52-1 Acid 2,4-Dinitrotoluene 121-14-2 Base-neutral Heptachlor 76-44-8 Base-neutral Hexachlorobenzene 118-74-1 Base-neutral Hexachlorobutadiene 87-68-3 Base-neutral Hexachloroethane 67-72-1 Base-neutral Hexachlorocyclopentadiene 77-47-4 Base-neutral Naphthalene 91-20-3 Base-neutral Nitrobenzene 98-95-3 Base-neutral 4-Nitrophenol 100-02-7 Acid Pentachlorophenol 87-86-5 Acid Phenol 108-95-2 Acid Phorate 298-02-2 Base-neutral 2-Picoline 109-06-8 Base-neutral Pyridine 110-86-1 Base-neutral Tetrachlorobenzene(s) Base-neutral Tetrachlorophenol(s) Acid Toxaphene 8001-35-2 Base-neutral Trichlorophenol(s) Acid 2,4,5-TP (Silvex) 93-72-1 Acid _______________________________________________________________________________ a Chemical Abstract Service Registry Number. -

Acids and Bases Experiments (Organic Classes)
Acids and Bases Experiments (Organic Classes) NaHCO3 (sodium bicarbonate, baking soda) and vinegar (dilute acetic acid) will be used to shoot a cork out of a bottle to show that some acid-base reactions generate gases like CO2. All students in the class will participate in a voice-activated chemical reaction. Students will remove a stopper and speak into a flask containing base and an indicator that will change color once enough CO2 is introduced from the students’ breath. Further color changes from basic to acidic conditions are illustrated using natural and household liquids and a universal indicator prepared from red cabbage juice. Finally, conversion between colorless and colored forms of different acid-base indicators in unusual settings will be shown in the “invisible painting” experiment. Supplies Needed: For the rocket reaction: You provide the following items indicated in red and starred: * empty 1 L plastic soda bottle with label removed * vinegar (colorless) Plastic powder funnel (we provide) cork with streamers attached by a thumbtack (we provide) container of de-ionized water (we provide, you refill as needed) sodium bicarbonate (we provide, return unused) Please clean and return all materials For the voice-activated chemical reaction: water that we provide as 250 mL Erlenmeyer flask (we provide)) soon as possible. dropper bottle with colorless phenolphtalein indicator (we provide) Other students may small amount of 1 M NaOH solution (we provide) need to use this “kit” disposable plastic droppers (we provide) for their ChemDemo!! For the universal pH indicator from red cabbage: * Red cabbage juice (you make the day before the demo) * 1 lemon, 1 soap bar, Sprite, vinegar, drain cleaner * 7 stirring rods (e.g. -

Estimating Ph at the Air/Water Interface with a Confocal
ANALYTICAL SCIENCES OCTOBER 2015, VOL. 31 1 2015 © The Japan Society for Analytical Chemistry Supporting Information Estimating pH at the Air/Water Interface with a Confocal Fluorescence Microscope Haiya YANG, Yasushi IMANISHI, Akira HARATA† Department of Molecular and Material Sciences, Interdisciplinary Graduate School of Engineering Sciences, Kyushu University, 6-1 Kasugakoen, Kasuga-shi, Fukuoka 816-8580, Japan † To whom correspondence should be addressed. Email: [email protected] 1 2 ANALYTICAL SCIENCES OCTOBER 2015, VOL. 31 Mathematical relationship between fluorescence peak wavenumbers and pH for a fluorescent pH indicator In a confocal fluorescence microscope, the probe volume is confined in an elongated cylindrical shape with radius and height . If the position of the surface is defined to be exactly at the symmetrical plane horizontally intersecting the cylinder, the probe area and probe volume are and for the surface observation, while they are zero and for the bulk observation, respectively. For a component i, the ratio of fluorescent intensity detected for the surface observation with respect to the bulk observation can be given as (S1) where and represent the efficiencies of fluorescence excitation detection per fluorescent molecule at the surface and in the bulk solution, respectively; is the surface density, and is the bulk concentration. Because , Eq. (S1) is deformed into ; (S2) when , a surface-selective observation for this surface-active component at the water surface is available.17 In this case, and at a low concentration limit, the pH-dependent fluorescence spectrum of the surface-adsorbed pH indictor is given by , (S3) 2 ANALYTICAL SCIENCES OCTOBER 2015, VOL. -

Ph (Colorimetric) See a Separate Application for the Arena Or Gallery Analyzer
Page 1 D10493_03_Insert_pH_Colorimetric EN TEST PROCEDURE pH (colorimetric) See a separate application for the Arena or Gallery analyzer. 984349 Materials required but not provided 3 x 20 ml Reagent 1 Distilled water (aseptic and free of heavy metals) and general laboratory equipment. INTENDED USE Reagent for photometric determination of pH (colorimetric) in Calibrators (pH 3, 4, 5, 6). homogenous liquid samples using automated Thermo Scientific Arena pH 4 Std for QC, Thermo Fisher Scientific Cat no 984331 or Gallery analyzer. pH 7 Std for QC, Thermo Fisher Scientific Cat no 984332 METHOD Calibration Colorimetric test with pH indicator dyes in an aqueous solution. This method has been developed using 4 separate calibration points. Method is performed at 37 °C, using 575 nm filter and 700 nm as side Calibrators used were: wavelenght. Fixanal pH 3, Fixanal pH 4, Fixanal pH 5 and Fixanal pH 6 from PRINCIPLE OF THE PROCEDURE Sigma-Aldrich. pH (colorimetric) method is based on the property of acid-base indicator dyes, which produce color depending on the pH of the Note: Samples measured with manual pH meter can also be used as sample. The color change can be measured as an absorbance calibrators. In this case the calibration must be performed with same change spectrophotometrically. matrix type and with several points covering the whole measuring range. This calibration type must be validated by the user. REAGENT INFORMATION Barcode ID Quality Control Reagent 1 (R1) 3 x 20 ml A13 Use quality control samples at least once a day and after each calibration and every time a new bottle of reagent is used. -
![I24 [No Document #]](https://docslib.b-cdn.net/cover/4696/i24-no-document-1474696.webp)
I24 [No Document #]
Petrographic Analysis of Concrete Core Samples from Saltstone Disposal Unit #6 at the Savannah River Site, Aiken, SC Robert D. Moser, E. Rae Gore, and Kyle L. Klaus March 2016 Laboratory Geotechnical and Structures and Geotechnical i Executive Summary: This study examined five concrete cores provided to the ERDC by the U.S. Department of Energy Savannah River Site from its Saltstone Disposal Unit #6 facility (SDU6). The five cores, which were logged in as CMB No. 160051-1 to 160051-5 which were removed from three different floor placement locations in the structure were subjected to an in-depth analysis consisting of visual and petrographic examination, electron microscopy, pH measurements, elemental mapping, and air void analysis. The results of the study indicated through depth cracks in the slabs that appeared to be driven by shrinkage. Additional analysis identified that smaller cracks less than 0.1 mm in width possible occurring at early ages in the concrete had largely self healed while larger through depth cracks with widths greater than 0.1 mm and in many cases greater than 0.5 mm remained. Many of these large cracks had been successfully infilled with epoxy with the exception of large voids which intersected the cracks. Cracks often intersected coarse aggregates, indicating that the cracks likely occurred at later ages when the concrete strength was high. No vertical displacement was observed between adjacent crack faces. pH indicator solution measurements made using optical microscopy indicated that high pH remains within the concrete even adjacent to cracks. The near surface concrete has reduced pH to depths of up to 2 mm caused by exposure to acidic water and carbonation. -

One Step Forward, NO Steps Back
E-Catalog READY-TO-USE ANALYTICAL SOLUTIONS & STANDARDS We have the right chemical testing solutions, ready when One step forward, you need them. As the largest independent manufacturer NO steps back or ready-to-use inorganic analytical solutions and standards in North America, our dedicated team of technical service Improved your development processes with solutions from RICCA CHEMICAL COMPANY experts will ensure that you receive the products you need in the lab, in the field and on the production floor. RIGHT • READY • RICCA CUSTOM CAPABILITIES READY-TO-USE ANALYTICAL SOLUTIONS & STANDARDS RICCA Chemical Company Custom Solutions Meet Your Special Specifications. Table of Contents Custom Capabilities Page 3 Buffers Page 4 Chemical Indicators Page 14 Titrants Page 30 Spectroscopy Reagents Page 60 Conductivity Standards Page 88 We’re ready to meet your every need for formulas, packaging, and labeling ISE Standards and Reagents Page 98 through our custom manufacturing capabilities. We can custom make any product you need. Some of our most Standards Page 102 Custom–Made Reagent Solutions and Formulations common products include: In Vitro Diagnostic Page 118 • Quality Control Chemists can meet your special test requirements • Buffers • Raw materials based on your specifications Reagent Grade Chemicals Page 128 • Standards • Manufactured precisely to your requirements • Acids, Bases, and Titrants Solvents Page 138 Custom–Made Batch Sizes • Spectroscopy/ICP Blends Compendial Reagents Page 142 • 1 ounce to almost 1,600 gallons are available -

Lab 4: Molecules in Biotechnology; Ph & Buffers; DNA Isolation From
Lab 4: Molecules in Biotechnology; pH & Buffers; DNA Isolation from Strawberries We will begin today’s lab with a lecture on cellular molecules in biotechnology. There are four main categories of cellular molecules: 1) Carbohydrates 2) Lipids 3) Proteins 4) Nucleic acids The lecture is in Powerpoint format, and I have printed the slides for you so that you can follow along and take additional notes. From this point on in the semester, be aware of each reagent that we use in lab and know what category of cellular molecule it falls into. Each category of cellular molecule has different biological and chemical properties, so it is always helpful to know the type of molecule with which you are working during an experiment. 34 35 36 37 38 Activity 4a Measuring the pH of Solutions Purpose In this activity you will learn about pH and test the pH of some common solutions. The pH of a solution can be extremely critical to its biological and chemical properties. Solvents of a specific pH are often necessary in biotechnology labs to prepare solutions of DNA, protein, nucleic acids, and other cellular molecules. Background Definition of pH: pH is a measurement of the concentration of hydrogen ions (= H+, or protons) in a solution. Numerically pH is defined as the negative logarithm of the concentration of H+ ions expressed in moles per liter (M). pH = -log [H+] Pure water spontaneously dissociates into ions, forming a 10-7 M solution of H+ (and OH-). The negative of this logarithm is 7, so the pH of pure water is 7. -

The Control and Measurement of 'CO 2' During Fermentations
Journal of Microbiological Methods 10 (1989) 155 -176 155 Elsevier MIMET 00338 Review Article The control and measurement of 'CO 2' during fermentations Neil M. Dixon and Douglas B. Kell Department of Biological Sciences, University College of Wales, Aberystwyth, Dyfed, UK (Received 6 June 1989; revision received 18 August 1989; accepted 28 August 1989) 1. Introduction It has been known for many years that the growth and metabolism of microorgan- isms is accompanied by the uptake and/or evolution of CO 2. To study and indeed to exploit the effects of CO2 on microbial metabolism, it is necessary to control the level of (dissolved) CO2 within the culture medium. Whilst several articles cover one or two methods by which pCO2 may be measured and, hence, controlled, we know of no review of these. Thus, the purpose of the present article is to provide an overview of the role, measurement and control of the magnitude ofpCO2 during laboratory and industrial fermentations. We begin by describing the various 'CO 2' equilibria and the question of CO2-absorption rates. 2. 'C02' Concentrations Although the partial pressure of CO 2 in the gas phase may be held constant, the ratios of the different possible species of 'CO2' in the aqueous phase will vary as a function of the pH and other factors. Since CO2 can hydrate and dissociate in water, the reaction scheme may be written [1] as: CO 2 + H20 ~ H2CO 3 .-" HCO~ + H + '-" CO 2- + 2H + (1) In addition, it has recently been proposed that small concentrations of dimeric hydrogen carbonate ions (H3C20~-) exist near neutral pH [2]. -

The Rainbow in a Bottle Reaction Can You Make Your Very Own Rainbow in a Bottle? You Can with the Magic of Ph! Ph Is a Measurement of How Acidic Or Basic Something Is
Chemistry The Rainbow in a Bottle Reaction Can you make your very own rainbow in a bottle? You can with the magic of pH! pH is a measurement of how acidic or basic something is. Very acidic things have a pH of 0, while very basic things have a pH of 14. What’s in the middle? Neutral materials, like water, have a pH of around 7. Red grape juice naturally changes color base on pH, so we can use it as a pH indicator. Grape juice is mostly made out of the water and is fairly neutral, so its purple color indicates around pH 7. An acid with pH 0 would be a bright clear yellow. A base with a very high pH will have a green color. We can make a rainbow by first making an acid solution and then slowly lowering the pH until the solution is a base. We can make that happen slowly with a medicine called milk of magnesia, which can neutralize acids. A Water Bottle 1 cup - water 2 TBS - red grape juice 2 TBS - vinegar Supplies 2 TBS - milk of magnesia Other household chemicals Can you make a Rainbow in a Bottle? 1. Fill the bottle with about 1 cup of water, that’s about half of a small water bottle. 2. Add 2 TBS of Red Grape Juice. Add 2 TBS of vinegar. 3. Add 2 TBS of Milk of Magnesia. Challenge 4. Put the lid on the bottle tightly and shake vigorously. Guiding Questions? 1. What color do you see when you add each chemical to the bottle? 2. -

Analysis of the Diffusion Process by Ph Indicator in Microfluidic Chips
micromachines Article Analysis of the Diffusion Process by pH Indicator in Microfluidic Chips for Liposome Production Elisabetta Bottaro 1,2 , Ali Mosayyebi 1, Dario Carugo 1,3,* and Claudio Nastruzzi 2,* 1 Bioengineering Science Group, Faculty of Engineering and the Environment, University of Southampton, Southampton SO17 1BJ, UK; [email protected] (E.B.); [email protected] (A.M.) 2 Department of Life Science and Biotechnology, University of Ferrara, Via Fossato di Mortara 17, 44121 Ferrara, Italy 3 Institute for Life Sciences (IfLS), University of Southampton, Southampton SO17 1BJ, UK * Correspondences: [email protected] (D.C.); [email protected] (C.N.); Tel.: +44-(0)23-8059-3242 (D.C.); +39-0532-455348 (C.N.) Received: 28 April 2017; Accepted: 17 June 2017; Published: 1 July 2017 Abstract: In recent years, the development of nano- and micro-particles has attracted considerable interest from researchers and enterprises, because of the potential utility of such particles as drug delivery vehicles. Amongst the different techniques employed for the production of nanoparticles, microfluidic-based methods have proven to be the most effective for controlling particle size and dispersity, and for achieving high encapsulation efficiency of bioactive compounds. In this study, we specifically focus on the production of liposomes, spherical vesicles formed by a lipid bilayer encapsulating an aqueous core. The formation of liposomes in microfluidic devices is often governed by diffusive mass transfer of chemical species at the liquid interface between a solvent (i.e., alcohol) and a non-solvent (i.e., water). In this work, we developed a new approach for the analysis of mixing processes within microfluidic devices.