Some Chytrids of Taiwan (I)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

For Review Only 377 Algomyces Stechlinensis Clustered Together with Environmental Clones from a Eutrophic 378 Lake in France (Jobard Et Al
Journal of Eukaryotic Microbiology Page 18 of 43 1 Running head: Parasitic chytrids of volvocacean algae. 2 3 Title: Diversity and Hidden Host Specificity of Chytrids infecting Colonial 4 Volvocacean Algae. 5 Authors: Silke Van den Wyngaerta, Keilor Rojas-Jimeneza,b, Kensuke Setoc, Maiko Kagamic, 6 Hans-Peter Grossarta,d 7 a Department of ExperimentalFor Limnology, Review Leibniz-Institute Only of Freshwater Ecology and Inland 8 Fisheries, Alte Fischerhuette 2, D-16775 Stechlin, Germany 9 b Universidad Latina de Costa Rica, Campus San Pedro, Apdo. 10138-1000, San Jose, Costa Rica 10 c Department of Environmental Sciences, Faculty of Science, Toho University, Funabashi, Chiba, 11 Japan 12 d Institute of Biochemistry and Biology, Potsdam University, Maulbeerallee 2, 14476 Potsdam, 13 Germany 14 15 Corresponding Author: 16 Silke Van den Wyngaert, Department of Experimental Limnology, Leibniz-Institute of 17 Freshwater Ecology and Inland Fisheries, Alte Fischerhuette 2, D-16775 Stechlin, Germany 18 Telephone number: +49 33082 69972; Fax number: +49 33082 69917; e-mail: [email protected], 19 [email protected] 20 21 22 23 1 Page 19 of 43 Journal of Eukaryotic Microbiology 24 ABSTRACT 25 Chytrids are zoosporic fungi that play an important, but yet understudied, ecological role in 26 aquatic ecosystems. Many chytrid species have been morphologically described as parasites on 27 phytoplankton. However, the majority of them have rarely been isolated and lack DNA sequence 28 data. In this study we isolated and cultivated three parasitic chytrids, infecting a common 29 volvocacean host species, Yamagishiella unicocca. In order to identify the chytrids, we 30 characterized morphology and life cycle, and analyzed phylogenetic relationships based on 18S 31 and 28S rDNA genes. -

The Classification of Lower Organisms
The Classification of Lower Organisms Ernst Hkinrich Haickei, in 1874 From Rolschc (1906). By permission of Macrae Smith Company. C f3 The Classification of LOWER ORGANISMS By HERBERT FAULKNER COPELAND \ PACIFIC ^.,^,kfi^..^ BOOKS PALO ALTO, CALIFORNIA Copyright 1956 by Herbert F. Copeland Library of Congress Catalog Card Number 56-7944 Published by PACIFIC BOOKS Palo Alto, California Printed and bound in the United States of America CONTENTS Chapter Page I. Introduction 1 II. An Essay on Nomenclature 6 III. Kingdom Mychota 12 Phylum Archezoa 17 Class 1. Schizophyta 18 Order 1. Schizosporea 18 Order 2. Actinomycetalea 24 Order 3. Caulobacterialea 25 Class 2. Myxoschizomycetes 27 Order 1. Myxobactralea 27 Order 2. Spirochaetalea 28 Class 3. Archiplastidea 29 Order 1. Rhodobacteria 31 Order 2. Sphaerotilalea 33 Order 3. Coccogonea 33 Order 4. Gloiophycea 33 IV. Kingdom Protoctista 37 V. Phylum Rhodophyta 40 Class 1. Bangialea 41 Order Bangiacea 41 Class 2. Heterocarpea 44 Order 1. Cryptospermea 47 Order 2. Sphaerococcoidea 47 Order 3. Gelidialea 49 Order 4. Furccllariea 50 Order 5. Coeloblastea 51 Order 6. Floridea 51 VI. Phylum Phaeophyta 53 Class 1. Heterokonta 55 Order 1. Ochromonadalea 57 Order 2. Silicoflagellata 61 Order 3. Vaucheriacea 63 Order 4. Choanoflagellata 67 Order 5. Hyphochytrialea 69 Class 2. Bacillariacea 69 Order 1. Disciformia 73 Order 2. Diatomea 74 Class 3. Oomycetes 76 Order 1. Saprolegnina 77 Order 2. Peronosporina 80 Order 3. Lagenidialea 81 Class 4. Melanophycea 82 Order 1 . Phaeozoosporea 86 Order 2. Sphacelarialea 86 Order 3. Dictyotea 86 Order 4. Sporochnoidea 87 V ly Chapter Page Orders. Cutlerialea 88 Order 6. -

Collecting and Recording Fungi
British Mycological Society Recording Network Guidance Notes COLLECTING AND RECORDING FUNGI A revision of the Guide to Recording Fungi previously issued (1994) in the BMS Guides for the Amateur Mycologist series. Edited by Richard Iliffe June 2004 (updated August 2006) © British Mycological Society 2006 Table of contents Foreword 2 Introduction 3 Recording 4 Collecting fungi 4 Access to foray sites and the country code 5 Spore prints 6 Field books 7 Index cards 7 Computers 8 Foray Record Sheets 9 Literature for the identification of fungi 9 Help with identification 9 Drying specimens for a herbarium 10 Taxonomy and nomenclature 12 Recent changes in plant taxonomy 12 Recent changes in fungal taxonomy 13 Orders of fungi 14 Nomenclature 15 Synonymy 16 Morph 16 The spore stages of rust fungi 17 A brief history of fungus recording 19 The BMS Fungal Records Database (BMSFRD) 20 Field definitions 20 Entering records in BMSFRD format 22 Locality 22 Associated organism, substrate and ecosystem 22 Ecosystem descriptors 23 Recommended terms for the substrate field 23 Fungi on dung 24 Examples of database field entries 24 Doubtful identifications 25 MycoRec 25 Recording using other programs 25 Manuscript or typescript records 26 Sending records electronically 26 Saving and back-up 27 Viruses 28 Making data available - Intellectual property rights 28 APPENDICES 1 Other relevant publications 30 2 BMS foray record sheet 31 3 NCC ecosystem codes 32 4 Table of orders of fungi 34 5 Herbaria in UK and Europe 35 6 Help with identification 36 7 Useful contacts 39 8 List of Fungus Recording Groups 40 9 BMS Keys – list of contents 42 10 The BMS website 43 11 Copyright licence form 45 12 Guidelines for field mycologists: the practical interpretation of Section 21 of the Drugs Act 2005 46 1 Foreword In June 2000 the British Mycological Society Recording Network (BMSRN), as it is now known, held its Annual Group Leaders’ Meeting at Littledean, Gloucestershire. -

Chytridiomycetes, Chytridiomycota)
VOLUME 5 JUNE 2020 Fungal Systematics and Evolution PAGES 17–38 doi.org/10.3114/fuse.2020.05.02 Taxonomic revision of the genus Zygorhizidium: Zygorhizidiales and Zygophlyctidales ord. nov. (Chytridiomycetes, Chytridiomycota) K. Seto1,2,3*, S. Van den Wyngaert4, Y. Degawa1, M. Kagami2,3 1Sugadaira Research Station, Mountain Science Center, University of Tsukuba, 1278-294, Sugadaira-Kogen, Ueda, Nagano 386-2204, Japan 2Department of Environmental Science, Faculty of Science, Toho University, 2-2-1, Miyama, Funabashi, Chiba 274-8510, Japan 3Graduate School of Environment and Information Sciences, Yokohama National University, 79-7, Tokiwadai, Hodogaya, Yokohama, Kanagawa 240- 8502, Japan 4Department of Experimental Limnology, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Alte Fischerhuette 2, D-16775 Stechlin, Germany *Corresponding author: [email protected] Key words: Abstract: During the last decade, the classification system of chytrids has dramatically changed based on zoospore Chytridiomycota ultrastructure and molecular phylogeny. In contrast to well-studied saprotrophic chytrids, most parasitic chytrids parasite have thus far been only morphologically described by light microscopy, hence they hold great potential for filling taxonomy some of the existing gaps in the current classification of chytrids. The genus Zygorhizidium is characterized by an zoospore ultrastructure operculate zoosporangium and a resting spore formed as a result of sexual reproduction in which a male thallus Zygophlyctis and female thallus fuse via a conjugation tube. All described species of Zygorhizidium are parasites of algae and Zygorhizidium their taxonomic positions remain to be resolved. Here, we examined morphology, zoospore ultrastructure, host specificity, and molecular phylogeny of seven cultures of Zygorhizidium spp. Based on thallus morphology and host specificity, one culture was identified as Z. -

1993 Li, Heath and Packer.Pdf
The phylogenetic relationships of the anaerobic chytridiomycetous gut fungi (Neocallimasticaceae) and the Chytridiornycota. 11. Cladistic analysis of structural data and description of Neocallimasticales ord.nov. JINLIANCLI, I. BRENTHEATH,] AND LAURENCEPACKER Dep(~rittiet~tof Biology, York Utliversity, North York, Otzt., Cotzodc~M3J IP3 Receivcd May 15, 1992 LI, J., HEATH,I. B., and PACKER,L. 1993. The phylogenetic relationships of the anaerobic chytridiomycetous gut fungi (Neocallimasticaceae) and the Chytridiornycota. 11. Cladistic analysis of structural data and description of Neocalli- masticales ord.nov. Can. J. Bot. 71: 393-407. We investigated the phylogenetic relationships of thc Chytridiomycota and the chytridiomycetous gut fungi with a cladistic analysis of42 morphological, ultrastructural, and mitotic characters for 38 taxa using both maximum parsimony and distance algorithms. Our analyses show that there are three major clades within the Chytridiomycota: the gut fungi, thc Blastocladiales, and the Spizellomycetales-Chytridialcs- Monoblepharidales. Conscqucntly. we elevated the gut fungi to the order Neocallimasticales ord.nov. Our results suggest that a modified Chytridiales, including the Monoblepharidales. is a monophyletic group. In contrast the Spizellomycetales are paraphyletic because the Chytridiales arose within them. The separation of the traditional Chytridiales into two orders is thus doubtful. Although the Blastocladiales are closer to members of the Spizellomycetales than the Chytridiales, the cladistic analyses of both structural and rRNA sequence data do not support the idea that the Blastocladiales were derived from the Spizellomycetales. We suggest emendations to the classification of the Chytridiomycota and note which groupings require further analysis. Our phylogeny for the currently recognized species of gut fungi is inconsis- tent with the existing classification. -

Clade (Kingdom Fungi, Phylum Chytridiomycota)
TAXONOMIC STATUS OF GENERA IN THE “NOWAKOWSKIELLA” CLADE (KINGDOM FUNGI, PHYLUM CHYTRIDIOMYCOTA): PHYLOGENETIC ANALYSIS OF MOLECULAR CHARACTERS WITH A REVIEW OF DESCRIBED SPECIES by SHARON ELIZABETH MOZLEY (Under the Direction of David Porter) ABSTRACT Chytrid fungi represent the earliest group of fungi to have emerged within the Kingdom Fungi. Unfortunately despite the importance of chytrids to understanding fungal evolution, the systematics of the group is in disarray and in desperate need of revision. Funding by the NSF PEET program has provided an opportunity to revise the systematics of chytrid fungi with an initial focus on four specific clades in the order Chytridiales. The “Nowakowskiella” clade was chosen as a test group for comparing molecular methods of phylogenetic reconstruction with the more traditional morphological and developmental character system used for classification in determining generic limits for chytrid genera. Portions of the 18S and 28S nrDNA genes were sequenced for isolates identified to genus level based on morphology to seven genera in the “Nowakowskiella” clade: Allochytridium, Catenochytridium, Cladochytrium, Endochytrium, Nephrochytrium, Nowakowskiella, and Septochytrium. Bayesian, parsimony, and maximum likelihood methods of phylogenetic inference were used to produce trees based on one (18S or 28S alone) and two-gene datasets in order to see if there would be a difference depending on which optimality criterion was used and the number of genes included. In addition to the molecular analysis, taxonomic summaries of all seven genera covering all validly published species with a listing of synonyms and questionable species is provided to give a better idea of what has been described and the morphological and developmental characters used to circumscribe each genus. -

Protista (PDF)
1 = Astasiopsis distortum (Dujardin,1841) Bütschli,1885 South Scandinavian Marine Protoctista ? Dingensia Patterson & Zölffel,1992, in Patterson & Larsen (™ Heteromita angusta Dujardin,1841) Provisional Check-list compiled at the Tjärnö Marine Biological * Taxon incertae sedis. Very similar to Cryptaulax Skuja Laboratory by: Dinomonas Kent,1880 TJÄRNÖLAB. / Hans G. Hansson - 1991-07 - 1997-04-02 * Taxon incertae sedis. Species found in South Scandinavia, as well as from neighbouring areas, chiefly the British Isles, have been considered, as some of them may show to have a slightly more northern distribution, than what is known today. However, species with a typical Lusitanian distribution, with their northern Diphylleia Massart,1920 distribution limit around France or Southern British Isles, have as a rule been omitted here, albeit a few species with probable norhern limits around * Marine? Incertae sedis. the British Isles are listed here until distribution patterns are better known. The compiler would be very grateful for every correction of presumptive lapses and omittances an initiated reader could make. Diplocalium Grassé & Deflandre,1952 (™ Bicosoeca inopinatum ??,1???) * Marine? Incertae sedis. Denotations: (™) = Genotype @ = Associated to * = General note Diplomita Fromentel,1874 (™ Diplomita insignis Fromentel,1874) P.S. This list is a very unfinished manuscript. Chiefly flagellated organisms have yet been considered. This * Marine? Incertae sedis. provisional PDF-file is so far only published as an Intranet file within TMBL:s domain. Diplonema Griessmann,1913, non Berendt,1845 (Diptera), nec Greene,1857 (Coel.) = Isonema ??,1???, non Meek & Worthen,1865 (Mollusca), nec Maas,1909 (Coel.) PROTOCTISTA = Flagellamonas Skvortzow,19?? = Lackeymonas Skvortzow,19?? = Lowymonas Skvortzow,19?? = Milaneziamonas Skvortzow,19?? = Spira Skvortzow,19?? = Teixeiromonas Skvortzow,19?? = PROTISTA = Kolbeana Skvortzow,19?? * Genus incertae sedis. -

Comparative Genomics of Chytrid Fungi Reveal Insights Into the Obligate
www.nature.com/scientificreports OPEN Comparative genomics of chytrid fungi reveal insights into the obligate biotrophic and Received: 4 February 2019 Accepted: 31 May 2019 pathogenic lifestyle of Synchytrium Published: xx xx xxxx endobioticum Bart T. L. H. van de Vossenberg1,2, Sven Warris 1, Hai D. T. Nguyen3, Marga P. E. van Gent-Pelzer1, David L. Joly 4, Henri C. van de Geest1, Peter J. M. Bonants1, Donna S. Smith5, C. André Lévesque 3 & Theo A. J. van der Lee1 Synchytrium endobioticum is an obligate biotrophic soilborne Chytridiomycota (chytrid) species that causes potato wart disease, and represents the most basal lineage among the fungal plant pathogens. We have chosen a functional genomics approach exploiting knowledge acquired from other fungal taxa and compared this to several saprobic and pathogenic chytrid species. Observations linked to obligate biotrophy, genome plasticity and pathogenicity are reported. Essential purine pathway genes were found uniquely absent in S. endobioticum, suggesting that it relies on scavenging guanine from its host for survival. The small gene-dense and intron-rich chytrid genomes were not protected for genome duplications by repeat-induced point mutation. Both pathogenic chytrids Batrachochytrium dendrobatidis and S. endobioticum contained the largest amounts of repeats, and we identifed S. endobioticum specifc candidate efectors that are associated with repeat-rich regions. These candidate efectors share a highly conserved motif, and show isolate specifc duplications. A reduced set of cell wall degrading enzymes, and LysM protein expansions were found in S. endobioticum, which may prevent triggering plant defense responses. Our study underlines the high diversity in chytrids compared to the well-studied Ascomycota and Basidiomycota, refects characteristic biological diferences between the phyla, and shows commonalities in genomic features among pathogenic fungi. -

Toward a Fully Resolved Fungal Tree of Life
Annual Review of Microbiology Toward a Fully Resolved Fungal Tree of Life Timothy Y. James,1 Jason E. Stajich,2 Chris Todd Hittinger,3 and Antonis Rokas4 1Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, Michigan 48109, USA; email: [email protected] 2Department of Microbiology and Plant Pathology, Institute for Integrative Genome Biology, University of California, Riverside, California 92521, USA; email: [email protected] 3Laboratory of Genetics, DOE Great Lakes Bioenergy Research Center, Wisconsin Energy Institute, Center for Genomic Science and Innovation, J.F. Crow Institute for the Study of Evolution, University of Wisconsin–Madison, Madison, Wisconsin 53726, USA; email: [email protected] 4Department of Biological Sciences, Vanderbilt University, Nashville, Tennessee 37235, USA; email: [email protected] Annu. Rev. Microbiol. 2020. 74:291–313 Keywords First published as a Review in Advance on deep phylogeny, phylogenomic inference, uncultured majority, July 13, 2020 classification, systematics The Annual Review of Microbiology is online at micro.annualreviews.org Abstract https://doi.org/10.1146/annurev-micro-022020- Access provided by Vanderbilt University on 06/28/21. For personal use only. In this review, we discuss the current status and future challenges for fully 051835 Annu. Rev. Microbiol. 2020.74:291-313. Downloaded from www.annualreviews.org elucidating the fungal tree of life. In the last 15 years, advances in genomic Copyright © 2020 by Annual Reviews. technologies have revolutionized fungal systematics, ushering the field into All rights reserved the phylogenomic era. This has made the unthinkable possible, namely ac- cess to the entire genetic record of all known extant taxa. -

T. W. Johnson, Jr. Department of Botany, Duke Ur::.Iversit.Y and Di.A.Ne Testrake Wagner Biological Sciences, University of Smjt
THE AQUATIC FUNGI OF THE LAKE ITASCA REGION T. w. Johnson, Jr. Department of Botany, Duke Ur::.iversit.y and Di.a.ne TeStrake Wagner Biological Sciences, University of SmJth Florida California State College, Fullerton (on leave) Duke University September, 1967 \ ACKNOWLEDGMENTS This compilation is not solely the result of collections by the authors, although they are-responsible for the determinations. We acknowledge with gratitude the materials provided by Messrs. Baker, Colingsworth, Granovsky, Hobbs, and Tainter. Particular thanks are extended to David Padgett for many collections, particularly of the leptomitaceous species, and to Stephen Tarapchek for so graciously providing samples from the Red Lake Bog region. We are grateful for the facilities provided by, a.nd the cooperation of, the personnel of the Lake Itasca Forestry and Biological Station, University of Minnesota, and especially to its Associate Director, Dr. David French. The laborious task of preparing final copy fell to Mrs. Patricia James, Administrative Secretary, Department of Botany, Duke University. To her our special thanks. .,. 1 INTRODUCTION ~nis account is a first attempt at compiling an annotated list of aquatic fungi of the Lake Itasca region. As such, it is in complete (as all compilations subsequently prove to be), hence its intent is merely to provide a guide to those species encountered. Future collections undoubtedly will yield many species suspected to occur in this region but which are as yet uncovered. The aquatic fungi are a notoriously difficult group because of their ephemeral nature and the paucity of precise information about them. As a group, they embrace a wide diversity of forms, from the unspecialized unicell that converts entirely into a single reproductive unit to the extensive, mycelial type of growth in which many reproductive centers are formed~ Although there is great morphological variation, there is also a degree of remarkable similarity--even among representatives of separate and distinct orders. -
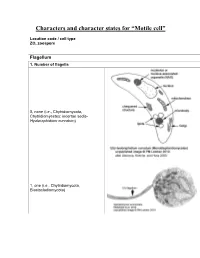
Characters and Character States for “Motile Cell”
Characters and character states for “Motile cell” Location code / cell type ZO, zoospore Flagellum 1. Number of flagella 0, none (i.e., Chytridiomycota, Chytridiomycetes: incertae sedis- Hyaloraphidium curvatum) 1, one (i.e., Chytridiomycota, Blastocladiomycota) 2, multiple (i.e., Neocallimastigomycota) 2. Electron-opaque plug in axoneme core and between axoneme and flagellar membrane 0, absent 1, present (i.e., Chytridiomycota: Chytridiomycetes: Chytridiales, Lobulomycetales, Cladochytriales, incertae sedis: Synchytrium endobioticum, Polychytrium clade; Monoblepharidomycetes) 3. Flagellum coating 0, absent (all taxa except Polyphagus euglenae) 1, present (Polyphagus euglenae) Kinetosome (the term “basal body” is synonymous; Andersen et al. 1991) 4. Electron-opaque core in kinetosome 0, absent (all taxa except Kappamyces) 1, present (Chytridiomycota: Chytridiomycetes: Rhizophydiales- Kappamyces) 5. Scalloped ring within kinetosome, extensions of the A, B, or C microtubule 0, absent 1, present (Lacustromyces hiemalis) Kinetosome-associated structures 6. Kinetosome support 0, absent (Thalassochytrium gracilaripsidis) 1, kinetosome props 2, broken kinetosome props (Olpidium radicale) 3, skirt-like structure surrounding kinetosome (Neocallimastigomycota) 7. Kinetosome-associated plates 0, absent 1, present (Chytridiomycota: Chytridiomycetes: Chytridiales- Group I- type zoospore [Barr 1980]) 8. Kinetosome-associated saddle 0, absent 1, present (Chytridiomycota: Chytridiomycetes: Chytridiales- Group II-type zoospore [Barr 1980]- Chytridium -

Phylogeny and Evolutionary Perspective of Opisthokonta Protists
Phylogeny and evolutionary perspective of Opisthokonta protists Guifré Torruella i Cortés ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tdx.cat) i a través del Dipòsit Digital de la UB (diposit.ub.edu) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX ni al Dipòsit Digital de la UB. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX o al Dipòsit Digital de la UB (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tdx.cat) y a través del Repositorio Digital de la UB (diposit.ub.edu) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. No se autoriza su reproducción con finalidades de lucro ni su difusión y puesta a disposición desde un sitio ajeno al servicio TDR o al Repositorio Digital de la UB.