SQUAMATA: COLUBRIDAE Opheodrys Aestivus (Linnaeus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blackwater NWR Reptiles and Amphibians List
U.S. Fish & Wildlife Service Blackwater National Wildlife Refuge 2145 Key Wallace Dr. Cambridge, MD 21613 410/228 2677 Fax: 410/221 7738 Blackwater Email: [email protected] http://www.fws.gov/blackwater/ National Wildlife U.S. Fish & Wildlife Service http://www.fws.gov Refuge For Refuge Information 1 800/344 WILD Reptiles & Amphibians Federal Relay Service for the deaf and hard-of-hearing 1 800/877 8339 Voice and TTY July 2008 NT OF E TH TM E R IN A P T E E R D I . O S R . U M A 49 RC H 3, 18 Northern Redbelly Turtle Rachel Woodward/ USFWS Reptiles The vast marshes and Reptiles are cold-blooded vertebrates including turtles, snakes and lizards. Reptiles are characterized by bodies bordering swamps of with dry skin (not slimy) and scales, Blackwater National or scutes. They usually lay eggs. Turtles Northern Redbelly Turtle Wildlife Refuge offer (Pseudemys rubriventris), Common, 10-12.5". Has a smooth, elongated shell that is olive-brown to black with ideal living conditions red vertical forked lines. Prefers larger bodies of fresh water and for an array of reptiles basks like the smaller painted turtle. and amphibians. These Largely vegetarian. Eastern Painted Turtle (Chrysemys p. picta), Common, cold-blooded animals 4-7". The most visible turtle on the refuge can be seen in the summer become dormant in and fall basking on logs in both fresh and brackish water. Has a smooth, flattened, olive to black shell with winter, but as spring yellow borders on seams. Limbs and tail are black with red stripes. -
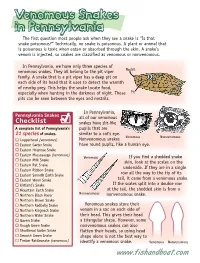
Venomous Snakes in Pennsylvania the First Question Most People Ask When They See a Snake Is “Is That Snake Poisonous?” Technically, No Snake Is Poisonous
Venomous Snakes in Pennsylvania The first question most people ask when they see a snake is “Is that snake poisonous?” Technically, no snake is poisonous. A plant or animal that is poisonous is toxic when eaten or absorbed through the skin. A snake’s venom is injected, so snakes are classified as venomous or nonvenomous. In Pennsylvania, we have only three species of venomous snakes. They all belong to the pit viper Nostril family. A snake that is a pit viper has a deep pit on each side of its head that it uses to detect the warmth of nearby prey. This helps the snake locate food, especially when hunting in the darkness of night. These Pit pits can be seen between the eyes and nostrils. In Pennsylvania, Pennsylvania Snakes all of our venomous Checklist snakes have slit-like A complete list of Pennsylvania’s pupils that are 21 species of snakes. similar to a cat’s eye. Venomous Nonvenomous Copperhead (venomous) Nonvenomous snakes Eastern Garter Snake have round pupils, like a human eye. Eastern Hognose Snake Eastern Massasauga (venomous) Venomous If you find a shedded snake Eastern Milk Snake skin, look at the scales on the Eastern Rat Snake underside. If they are in a single Eastern Ribbon Snake Eastern Smooth Earth Snake row all the way to the tip of its Eastern Worm Snake tail, it came from a venomous snake. Kirtland’s Snake If the scales split into a double row Mountain Earth Snake at the tail, the shedded skin is from a Northern Black Racer Nonvenomous nonvenomous snake. -

Smooth Green Snake
Smooth Green Snake Liochlorophis vernalis Taxa: Reptilian SE-GAP Spp Code: rSGSN Order: Squamata ITIS Species Code: 563910 Family: Colubridae NatureServe Element Code: ARADB47010 KNOWN RANGE: PREDICTED HABITAT: P:\Proj1\SEGap P:\Proj1\SEGap Range Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Range_rSGSN.pdf Predicted Habitat Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Dist_rSGSN.pdf GAP Online Tool Link: http://www.gapserve.ncsu.edu/segap/segap/index2.php?species=rSGSN Data Download: http://www.basic.ncsu.edu/segap/datazip/region/vert/rSGSN_se00.zip PROTECTION STATUS: Reported on March 14, 2011 Federal Status: --- State Status: CT (SC), IA (S), ID (P), IN (SE), MA (- WL), NC (W4,SC), ND (Level I), NE (NC), NH (SC), NJ (U), NY (GN), OH (SC), RI (Not Listed), TX (T), UT (SPC), QC (Susceptible) NS Global Rank: G5 NS State Rank: CO (S4), CT (S3S4), IA (S3), ID (SH), IL (S3S4), IN (S2), KS (SNA), MA (S5), MD (S5), ME (S5), MI (S5), MN (SNR), MO (SX), MT (S2), NC (SNA), ND (SNR), NE (S1), NH (S3), NJ (S3), NM (S4), NY (S4), OH (S4), PA (S3S4), RI (S5), SD (S4), TX (S1), UT (S2), VA (S3), VT (S3), WI (S4), WV (S5), WY (S2), MB (S3S4), NB (S5), NS (S5), ON (S4), PE (S3), QC (S3S4), SK (S3) rSGSN Page 1 of 4 SUMMARY OF PREDICTED HABITAT BY MANAGMENT AND GAP PROTECTION STATUS: US FWS US Forest Service Tenn. Valley Author. US DOD/ACOE ha % ha % ha % ha % Status 1 0.0 0 17.6 < 1 0.0 0 0.0 0 Status 2 0.0 0 1,117.9 < 1 0.0 0 0.0 0 Status 3 0.0 0 6,972.8 1 0.0 0 0.0 0 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 0.0 0 8,108.3 2 0.0 0 0.0 0 US Dept. -

Checklist Reptile and Amphibian
To report sightings, contact: Natural Resources Coordinator 980-314-1119 www.parkandrec.com REPTILE AND AMPHIBIAN CHECKLIST Mecklenburg County, NC: 66 species Mole Salamanders ☐ Pickerel Frog ☐ Ground Skink (Scincella lateralis) ☐ Spotted Salamander (Rana (Lithobates) palustris) Whiptails (Ambystoma maculatum) ☐ Southern Leopard Frog ☐ Six-lined Racerunner ☐ Marbled Salamander (Rana (Lithobates) sphenocephala (Aspidoscelis sexlineata) (Ambystoma opacum) (sphenocephalus)) Nonvenomous Snakes Lungless Salamanders Snapping Turtles ☐ Eastern Worm Snake ☐ Dusky Salamander (Desmognathus fuscus) ☐ Common Snapping Turtle (Carphophis amoenus) ☐ Southern Two-lined Salamander (Chelydra serpentina) ☐ Scarlet Snake1 (Cemophora coccinea) (Eurycea cirrigera) Box and Water Turtles ☐ Black Racer (Coluber constrictor) ☐ Three-lined Salamander ☐ Northern Painted Turtle ☐ Ring-necked Snake (Eurycea guttolineata) (Chrysemys picta) (Diadophis punctatus) ☐ Spring Salamander ☐ Spotted Turtle2, 6 (Clemmys guttata) ☐ Corn Snake (Pantherophis guttatus) (Gyrinophilus porphyriticus) ☐ River Cooter (Pseudemys concinna) ☐ Rat Snake (Pantherophis alleghaniensis) ☐ Slimy Salamander (Plethodon glutinosus) ☐ Eastern Box Turtle (Terrapene carolina) ☐ Eastern Hognose Snake ☐ Mud Salamander (Pseudotriton montanus) ☐ Yellow-bellied Slider (Trachemys scripta) (Heterodon platirhinos) ☐ Red Salamander (Pseudotriton ruber) ☐ Red-eared Slider3 ☐ Mole Kingsnake Newts (Trachemys scripta elegans) (Lampropeltis calligaster) ☐ Red-spotted Newt Mud and Musk Turtles ☐ Eastern Kingsnake -

Amphibians & Reptiles
AmphibiansAmphibians && ReptilesReptiles onon thethe NiagaraNiagara EscarpmentEscarpment By Fiona Wagner For anyone fortunate enough to be hiking the Bruce Trail in the early spring, it can be an overwhelming and humbling experience. That’s when the hills awaken to the resounding sound of frogs - the Wood frogs come first, followed by the Spring Peepers and Spiny Softshell Turtle Chrous frogs. By summer, seven more species will have joined this wondrous and sometimes deafening cacophony and like instruments in 15 an orchestra, each one has their unique call. Photo: Don Scallen Bruce Trail Magazine Spring 2010 Photo: Gary Hall Like an oasis in a desert of urban Toronto Zoo. “It’s this sheer diversi- of some of these creatures can be an development, the Niagara ty and the density of some of these astonishing experience, says Don Escarpment is home to more than experiences. I’m hooked for that Scallen, a teacher and vice president 30 reptiles and amphibians, includ- reason. You’ll never know what of Halton North Peel Naturalist ing several at risk species such as the you’re going to find.” Club. Don hits the Trail in early Dusky Salamander and Spotted spring to watch the rare and local Turtle (both endangered) as well as When and where to go Jefferson salamanders and their the threatened Jefferson Salamander. While it’s possible to see amphib- more common relative, the Yellow It is the diversity of the escarpment’s ians and reptiles from March to Spotted salamander. varied habitats, including wetlands, October, you’ll have more success if “A good night for salamander rocky outcrops and towering old you think seasonally, says Johnson. -

Snakes of the Everglades Agricultural Area1 Michelle L
CIR1462 Snakes of the Everglades Agricultural Area1 Michelle L. Casler, Elise V. Pearlstine, Frank J. Mazzotti, and Kenneth L. Krysko2 Background snakes are often escapees or are released deliberately and illegally by owners who can no longer care for them. Snakes are members of the vertebrate order Squamata However, there has been no documentation of these snakes (suborder Serpentes) and are most closely related to lizards breeding in the EAA (Tennant 1997). (suborder Sauria). All snakes are legless and have elongated trunks. They can be found in a variety of habitats and are able to climb trees; swim through streams, lakes, or oceans; Benefits of Snakes and move across sand or through leaf litter in a forest. Snakes are an important part of the environment and play Often secretive, they rely on scent rather than vision for a role in keeping the balance of nature. They aid in the social and predatory behaviors. A snake’s skull is highly control of rodents and invertebrates. Also, some snakes modified and has a great degree of flexibility, called cranial prey on other snakes. The Florida kingsnake (Lampropeltis kinesis, that allows it to swallow prey much larger than its getula floridana), for example, prefers snakes as prey and head. will even eat venomous species. Snakes also provide a food source for other animals such as birds and alligators. Of the 45 snake species (70 subspecies) that occur through- out Florida, 23 may be found in the Everglades Agricultural Snake Conservation Area (EAA). Of the 23, only four are venomous. The venomous species that may occur in the EAA are the coral Loss of habitat is the most significant problem facing many snake (Micrurus fulvius fulvius), Florida cottonmouth wildlife species in Florida, snakes included. -
Wildlife in Your Young Forest.Pdf
WILDLIFE IN YOUR Young Forest 1 More Wildlife in Your Woods CREATE YOUNG FOREST AND ENJOY THE WILDLIFE IT ATTRACTS WHEN TO EXPECT DIFFERENT ANIMALS his guide presents some of the wildlife you may used to describe this dense, food-rich habitat are thickets, T see using your young forest as it grows following a shrublands, and early successional habitat. timber harvest or other management practice. As development has covered many acres, and as young The following lists focus on areas inhabited by the woodlands have matured to become older forest, the New England cottontail (Sylvilagus transitionalis), a rare amount of young forest available to wildlife has dwindled. native rabbit that lives in parts of New York east of the Having diverse wildlife requires having diverse habitats on Hudson River, and in parts of Connecticut, Rhode Island, the land, including some young forest. Massachusetts, southern New Hampshire, and southern Maine. In this region, conservationists and landowners In nature, young forest is created by floods, wildfires, storms, are carrying out projects to create the young forest and and beavers’ dam-building and feeding. To protect lives and shrubland that New England cottontails need to survive. property, we suppress floods, fires, and beaver activities. Such projects also help many other kinds of wildlife that Fortunately, we can use habitat management practices, use the same habitat. such as timber harvests, to mimic natural disturbance events and grow young forest in places where it will do the most Young forest provides abundant food and cover for insects, good. These habitat projects boost the amount of food reptiles, amphibians, birds, and mammals. -

ES Teacher Packet.Indd
PROCESS OF EXTINCTION When we envision the natural environment of the Currently, the world is facing another mass extinction. past, one thing that may come to mind are vast herds However, as opposed to the previous five events, and flocks of a great diversity of animals. In our this extinction is not caused by natural, catastrophic modern world, many of these herds and flocks have changes in environmental conditions. This current been greatly diminished. Hundreds of species of both loss of biodiversity across the globe is due to one plants and animals have become extinct. Why? species — humans. Wildlife, including plants, must now compete with the expanding human population Extinction is a natural process. A species that cannot for basic needs (air, water, food, shelter and space). adapt to changing environmental conditions and/or Human activity has had far-reaching effects on the competition will not survive to reproduce. Eventually world’s ecosystems and the species that depend on the entire species dies out. These extinctions may them, including our own species. happen to only a few species or on a very large scale. Large scale extinctions, in which at least 65 percent of existing species become extinct over a geologically • The population of the planet is now growing by short period of time, are called “mass extinctions” 2.3 people per second (U.S. Census Bureau). (Leakey, 1995). Mass extinctions have occurred five • In mid-2006, world population was estimated to times over the history of life on earth; the first one be 6,555,000,000, with a rate of natural increase occurred approximately 440 million years ago and the of 1.2%. -

Nerodia Taxispilota)
ECOLOGY AND LIFE HISTORY OF THE BROWN WATER SNAKE (NERODIA TAXISPILOTA) by MARK S. MILLS (Under the direction of Dr. J. Whitfield Gibbons) ABSTRACT Population parameters, habitat, diet, reproductive traits, and other natural history characteristics of the brown water snake, Nerodia taxispilota, from the Savannah River Site, South Carolina, USA, were determined or estimated using mark-recapture data collected over an 8-yr period (1991-1998). Population size estimates for a 10-km section of the Savannah River ranged from 2782 - 3956 (approximately 0.14 - 0.20 snakes/m of shoreline). Growth was similar in juveniles of both sexes, but adult females grew significantly faster than adult males. Life history traits for this population include: 1) relatively high adult survivorship, 2) estimated ages at maturity of approximately 5-6 years for females and 3 years for males, 3) relatively long-lived (6+yr) individuals, 4) high fecundity (mean litter size =18.2), and 5) annual reproduction by females larger than 115 cm SVL. Litter size was positively correlated with female length and mass. No apparent trade-off exists between litter size and offspring size. Brown water snakes were not randomly distributed and were significantly associated with the steep-banked outer bends of the river and availability of potential perch sites. River sections with the highest number of captures were clustered within 200 m of backwater areas. Most (70%) of 164 recaptured N. taxispilota were <250 m from their previous capture site; however, three moved >1 km. Only large (>80 cm snout-vent length) individuals (n = 8) crossed the river (approximately 100 m). -

Amphibians and Reptiles of the State of Coahuila, Mexico, with Comparison with Adjoining States
A peer-reviewed open-access journal ZooKeys 593: 117–137Amphibians (2016) and reptiles of the state of Coahuila, Mexico, with comparison... 117 doi: 10.3897/zookeys.593.8484 CHECKLIST http://zookeys.pensoft.net Launched to accelerate biodiversity research Amphibians and reptiles of the state of Coahuila, Mexico, with comparison with adjoining states Julio A. Lemos-Espinal1, Geoffrey R. Smith2 1 Laboratorio de Ecología-UBIPRO, FES Iztacala UNAM. Avenida los Barrios 1, Los Reyes Iztacala, Tlalnepantla, edo. de México, Mexico – 54090 2 Department of Biology, Denison University, Granville, OH, USA 43023 Corresponding author: Julio A. Lemos-Espinal ([email protected]) Academic editor: A. Herrel | Received 15 March 2016 | Accepted 25 April 2016 | Published 26 May 2016 http://zoobank.org/F70B9F37-0742-486F-9B87-F9E64F993E1E Citation: Lemos-Espinal JA, Smith GR (2016) Amphibians and reptiles of the state of Coahuila, Mexico, with comparison with adjoining statese. ZooKeys 593: 117–137. doi: 10.3897/zookeys.593.8484 Abstract We compiled a checklist of the amphibians and reptiles of the state of Coahuila, Mexico. The list com- prises 133 species (24 amphibians, 109 reptiles), representing 27 families (9 amphibians, 18 reptiles) and 65 genera (16 amphibians, 49 reptiles). Coahuila has a high richness of lizards in the genus Sceloporus. Coahuila has relatively few state endemics, but has several regional endemics. Overlap in the herpetofauna of Coahuila and bordering states is fairly extensive. Of the 132 species of native amphibians and reptiles, eight are listed as Vulnerable, six as Near Threatened, and six as Endangered in the IUCN Red List. In the SEMARNAT listing, 19 species are Subject to Special Protection, 26 are Threatened, and three are in Danger of Extinction. -

Okefenokee National Wildlife Refuge Amphibians, Fish, Mammals and Reptiles List
U.S. Fish & Wildlife Service Okefenokee National Wildlife Refuge Amphibians, Fish, Mammals and Reptiles List The Okefenokee swamp is covered with Mammals ___Seminole Bat cypress, blackgum, and bay forests (Lasiurus seminolus). A common bat of scattered throughout a flooded prairie ___Virginia Opossum the Okefenokee which is found hanging in made of grasses, sedges, and various (Didelphis virginiana pigna). Common on Spanish Moss during the day. the swamp edge and the islands within aquatic plants. The peripheral upland and ___Hoary Bat the almost 70 islands within the swamp the Swamp. A night prowler. “Pogo” is often seen by campers. (Lasiurus cinereus cinereus). This are forested with pine interspersed with yellowish-brown bat flies high in the air hardwood hammocks. Lakes of varying ___Southern Short-Tailed Shrew late at night and will hang in trees when sizes and depths, and floating sections (Barina carolinensis). A specimen was resting. It is the largest bat in the East of the peat bed, are also part of the found on Floyds Island June 12, 1921. It and eats mostly moths. Okefenokee terrain. kills its prey with poisonous saliva. ___Northern Yellow Bat People have left their mark on the swamp. ___Least Shrew (Lasiurus intermedius floridanus). A 12-mile long canal was dug into the (Cryptotus parva parva). Rarely seen but Apparently a rare species in the area. It eastern prairies in the 1890’s in a failed probably fairly common. Specimens have likes to feed in groups. attempt to drain the swamp. During the been found on several of the islands, on early 1900’s large amounts of timber were the swamp edge, and in the pine woods ___Evening Bat removed, so that very few areas of virgin around the swamp. -

References for Life History
Literature Cited Adler, K. 1979. A brief history of herpetology in North America before 1900. Soc. Study Amphib. Rept., Herpetol. Cir. 8:1-40. 1989. Herpetologists of the past. In K. Adler (ed.). Contributions to the History of Herpetology, pp. 5-141. Soc. Study Amphib. Rept., Contrib. Herpetol. no. 5. Agassiz, L. 1857. Contributions to the Natural History of the United States of America. 2 Vols. Little, Brown and Co., Boston. 452 pp. Albers, P. H., L. Sileo, and B. M. Mulhern. 1986. Effects of environmental contaminants on snapping turtles of a tidal wetland. Arch. Environ. Contam. Toxicol, 15:39-49. Aldridge, R. D. 1992. Oviductal anatomy and seasonal sperm storage in the southeastern crowned snake (Tantilla coronata). Copeia 1992:1103-1106. Aldridge, R. D., J. J. Greenshaw, and M. V. Plummer. 1990. The male reproductive cycle of the rough green snake (Opheodrys aestivus). Amphibia-Reptilia 11:165-172. Aldridge, R. D., and R. D. Semlitsch. 1992a. Female reproductive biology of the southeastern crowned snake (Tantilla coronata). Amphibia-Reptilia 13:209-218. 1992b. Male reproductive biology of the southeastern crowned snake (Tantilla coronata). Amphibia-Reptilia 13:219-225. Alexander, M. M. 1943. Food habits of the snapping turtle in Connecticut. J. Wildl. Manag. 7:278-282. Allard, H. A. 1945. A color variant of the eastern worm snake. Copeia 1945:42. 1948. The eastern box turtle and its behavior. J. Tenn. Acad. Sci. 23:307-321. Allen, W. H. 1988. Biocultural restoration of a tropical forest. Bioscience 38:156-161. Anonymous. 1961. Albinism in southeastern snakes. Virginia Herpetol. Soc. Bull.