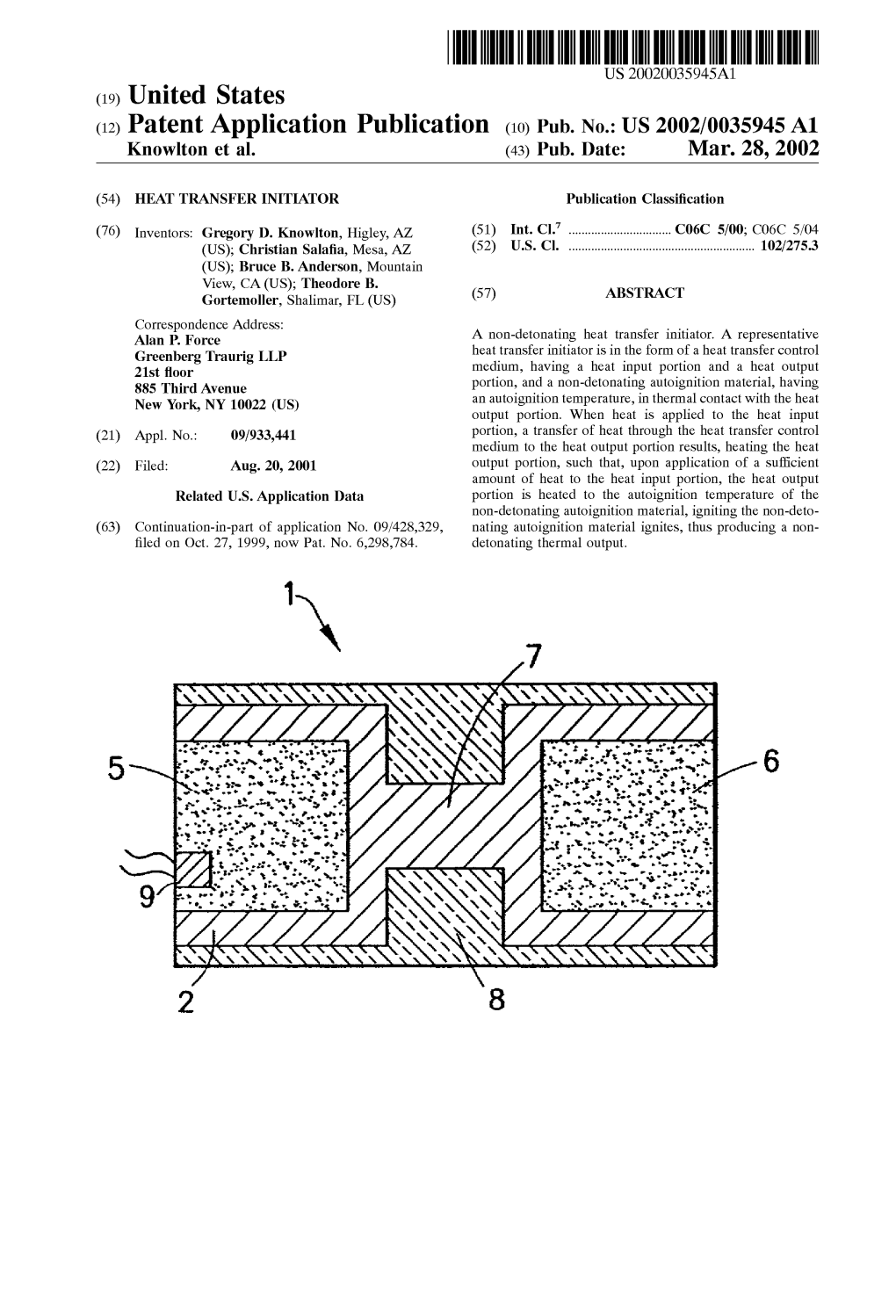
(12) Patent Application Publication (10) Pub. No.: US 2002/0035945A1 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermal Analysis and Stability of Boron/Potassium Nitrate
applied sciences Article Thermal Analysis and Stability of Boron/Potassium ◦ Nitrate Pyrotechnic Composition at 180 C Chaozhen Li 1 , Nan Yan 1,*, Yaokun Ye 2, Zhixing Lv 3, Xiang He 1, Jinhong Huang 4 and Nan Zhang 4 1 State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, China 2 Beijing Space Vehicle General Design Department, China Academy of Space Technology, Beijing 100094, China 3 Safety Technology Research Institute of Ordnance Industry, Beijing 100053, China 4 R & D Center, Liaoning North Huafeng Special Chemistry Co. Ltd., Fushun 113003, China * Correspondence: [email protected]; Tel.: +86-010-6891-2537 Received: 4 August 2019; Accepted: 29 August 2019; Published: 3 September 2019 Featured Application: This research aims to obtain the decomposition process of boron/potassium nitrate pyrotechnic composition, and verify its high temperature stability to meet the charge requirements of the separation device such as the cutter for the lunar probe. Abstract: Aerospace missions require that pyrotechnic compositions are able to withstand 180 ◦C. Therefore, this paper studies the thermal stability and output performance of boron/potassium nitrate (abbreviated BPN) used in pyrotechnic devices. Firstly, differential scanning calorimetry (DSC) and thermogravimetric (TG) tests are used to analyze the thermal reaction process of KNO3, boron, and BPN to qualitatively judge their thermal stability. Then, apparent morphology analysis, component analysis, and the p-t curve test, which is the closed bomb test to measure the output power of the pyrotechnic composition, are carried out with BPN samples before and after the high-temperature test to verify BPN stability at 180 ◦C. -

(12) Patent Application Publication (10) Pub. No.: US 2007/0068610 A1 Nickel (43) Pub
US 2007.006861 OA1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0068610 A1 Nickel (43) Pub. Date: Mar. 29, 2007 (54) MICROCRYSTALLINE NITROCELLULOSE Publication Classification PYROTECHNIC COMPOSITIONS (51) Int. Cl. (76) Inventor: Russell R. Nickel, Columbus, MT (US) C06B 45/10 (2006.01) (52) U.S. Cl. ............................................................ 149/19.8 Correspondence Address: PSERIEDER, WOODRUFF & (57) ABSTRACT 12412 POWERSCOURT DRIVE SUTE 200 ST. LOUIS, MO 63131-3615 (US) A. pyrotechnic composition comprising microcrystalline nitrocellulose which is characterized as an ultra low-smoke (21) Appl. No.: 11/469,936 composition. The pyrotechnic composition includes at least one flame coloring agent, and may be produced with or (22) Filed: Sep. 5, 2006 without an optional oxidizing agent, with or without an optional metal powder, with or without an optional chlorine Related U.S. Application Data donor. Upon combustion, the pyrotechnic composition pro duces illuminating emissions having desired colors and (63) Continuation-in-part of application No. 11/058,677, luminosity characteristics with significantly reduced or toxic filed on Feb. 15, 2005. combustion products and Smoke. US 2007/006861.0 A1 Mar. 29, 2007 MCROCRYSTALLINE NITROCELLULOSE may include at least one compound selected from a group PYROTECHNIC COMPOSITIONS consisting of ammonium perchlorate, alkali metal perchlo rates, alkali metal chlorates, alkali metal nitrates and alka CROSS-REFERENCE TO RELATED line earth metal nitrates. Additional fuels may include car APPLICATIONS bon, titanium, titanium alloys, Zirconium, Zirconium alloys, 0001. The present application is a continuation-in-part of iron, alloys of iron, magnesium, alloys of magnesium, U.S. patent application Ser. No. -

United States P Patented July 18, 1972
3,677,840 United States P Patented July 18, 1972 iodide of the invention is obtained in a highly active form 3,677,840 ideally suited for nucleating purposes. PYROTECHNICS COMPRISING OXDE OF SILVER The metathesis reaction proceeds substantially accord FOR WEATHERMODIFICATION USE ing to the following equation: Graham C. Shaw, Garland, and Russell Reed, Jr. Brigham City, Utah, assignors to Thiokol Chemical Corporation, Bristol, Pa. No Drawing. Filed Sept. 18, 1969, Ser. No. 859,165 In accordance with the invention, the pyrotechnic com int, C. C06d 3/00 o position comprises, by weight, the cured product produced U.S. C. 149-19 5 Claims by mixing and curing together from about 0.5% to about 10 20% of oxide of silver; from about 2% to about 45% of an alkali iodate present in about a stoichiometric amount ABSTRACT OF THE DISCLOSURE relative to the amount of oxide of silver present in the A pyrotechnic composition which upon combustion composition; from about 25% to about 75% of a solid in produces mixed silver halide nuclei for use in influencing organic oxidizer selected from the perchlorates and the weather comprises a composition made by curing a mix 5 nitrates of ammonium and of Group I-A and Group II-A ture comprising silver oxide, an alkali iodate, an alkali metals of the Periodic Table; and from about 10% to perchlorate and a curable oxygenated or fluorinated or about 20% of a curable, fluid polymer binder for pyro ganic liquid polymer binder. The composition burns technic compositions, especially a combined-halogen-rich smoothly to provide by metathesis a mixture of silver or combined-oxygen-rich polymer binder, preferably a halides as substantially the only solid or condensed phase 20 polyester-urethane terminated with amine or hydroxyl reaction products, and leaves substantially no residue. -

Manganese As Fuel in Slow-Burning Pyrotechnic Time Delay Compositions
Manganese as Fuel in Slow-Burning Pyrotechnic Time Delay Compositions 1 1 1∗ Darren Swanepoel , Olinto Del Fabbro and Walter W. Focke 1Institute of Applied Materials, Department of Chemical Engineering, University of Pretoria, Lynnwood Road, Pretoria, South Africa 2 † Corrie Conradie 2Research and Technology, African Explosives Limited, PO Modderfontein, 1645, South Africa Abstract Manganese metal was evaluated as a fuel for slow-burning delay compositions press- filled in aluminium or compaction-rolled in lead tubes. Oxides of antimony, bismuth, copper, manganese and vanadium were considered as oxidants. Measured burn rates for binary mixtures varied between 5 and 22 mm/s but slower burning ternary and quaternary compositions were also found. The addition of fumed silica to the Mn/MnO2 system had little effect on the propagation rate but a low level addition of hollow glass sphere significantly reduced the burn rate. Mn – MnO2 mixtures showed reliable burning over a wide stoichiometric range. In this system the fuel and the oxidant share a common metal. They combine to form the more stable intermediate oxide (MnO) releasing considerable quantities of heat in the process. Keywords: Pyrotechnics, Time delay, Manganese, Antimony oxide, Fumed silica 1 Introduction Commercial detonator delay element assemblies comprise an ignition source, a small- diameter tube containing a compacted pyrotechnic composition and an ignition transfer system [1, 2]. The pyrotechnic composition is a mixture of an oxidising agent and a fuel capable of an exothermic redox reaction. Following ignition, a combustion wave travels down along the tube at a constant velocity. This ensures the transmission of the initiation impulse to the detonator in a precisely adjustable time interval. -

Innovation Infosheet Thermite Torch Composition
Innovation Infosheet Downloaded October 2, 2021 Thermite Torch Composition Track Code: CRANE-97179 Categories: - Chemistry and Chemical Analysis - NSWC Crane Keywords: - Chemistry and Chemical Analysis - Crane - Demolition - Law Enforcement - Military - Welding Naval Surface Warfare Center, Crane Division (NSWC Crane) has developed and patented a series of compositions for thermite pyrotechnics which offer enhanced material perforation, increased reaction temperatures, and decreased toxicity. Thermite is a pyrotechnic composition of a metal powder and a metal oxide. When ignited, thermite produces an exothermic reaction with extremely high temperatures. These compositions are composed of magnalium, copper oxide, molybdenum, and a binder material. These three patents represent the optimal combination of components to enhance material perforation, improved gas production, temperature stability, heat transfer, shelf life, and low toxicity. Advantages: - Enhanced material perforation - Improved gas production - Temperature stability - Heat transfer - Longer shelf life - Decreased toxicity Potential Applications: - Disaster Clean-up - Law Enforcement - Mining - Metal Cutting and Welding - Demolition Downloaded October 2, 2021 Page 1 / 2 People: - D'Arche, Steve (Project leader) - Melof, Brian - Swanson, Travis Intellectual Property: Application Date: (None) Type: CON-Patent Country of Filing: United States Patent Number: 7,998,291 Issue Date: August 16, 2011 Application Date: (None) Type: CON-Patent Country of Filing: United States Patent Number: 7,988,802 Issue Date: August 2, 2011 Application Date: (None) Type: Utility Patent Country of Filing: United States Patent Number: 7,632,365 Issue Date: December 15, 2009 Contact OTC: Purdue Office of Technology Commercialization The Convergence Center 101 Foundry Drive, Suite 2500 West Lafayette, IN 47906 Phone: (765) 588-3475 Fax: (765) 463-3486 Email: [email protected] Downloaded October 2, 2021 Page 2 / 2. -

CHAPTER 5 Department of Agriculture
CHAPTER 5 Department of Agriculture Statutory Authority: 1976 Code §§ 23-39-90, 39-9-70, 39-9-80, 39-9-160, 39-11-20, 39-21-40, 39-27-60, 39-29-80, 39-31-100, 39-33-1230, 39-37-120, 39-39-40, 39-39-160, 39-41-80, 39-41-150, 46-15-20, 46-15-30, 46-21-20, 46-23-50 and 46-27-60; Chapters 11 and 25 of Title 39; Chapters 17, 19 (Article 5), 23 and 41 of Title 46; and Chapter 11 (Article 5) of Title 47 ARTICLE 1 AGRICULTURAL COMMODITIES MARKETING ACT SUBARTICLE 1 SOYBEANS A. MARKETING ORDER NO. 1 FOR SOUTH CAROLINA SOYBEANS 5–1. Definition of Terms. Terms used in this Marketing Order shall be as defined in the Act with the following additions: a. ‘‘Act’’ means the South Carolina Agricultural Commodities Marketing Act of 1968 and as amended in 1970. b. ‘‘Affected area’’ and ‘‘production area’’ are synonymous and mean the entire area of South Carolina. c. ‘‘Board’’ means the South Carolina Soybean Board established pursuant to the provisions of § 46-17-190 and 5-2 of this Marketing Order. d. ‘‘Bushel,’’ ‘‘Unit,’’ and ‘‘Affected unit’’ are synonymous and mean and include one (1) standard U. S. bushel of 60 pounds by weight of soybeans. e. ‘‘Commission’’ means the Agriculture Commission of South Carolina. f. ‘‘District’’ means the geographical divisions of the area of soybean production established pursuant to the provisions of 5-2 of this Marketing Order. g. ‘‘First buyer’’ means the person to whom soybeans are sold by the affected producer of said soybeans. -

APA STANDARD 87-1 Contents 1
APA STANDARD 87-1 Contents 1. INTRODUCTION..............................................................................................1 2. DEFINITIONS.....................................................................................................1 3. REQUIREMENTS FOR CONSUMER FIREWORKS, NOVELTIES AND THEATRICAL PYROTECHNICS .....................................................................4 3.1 Types of Consumer Fireworks.......................................................................5 3.2 Types of Novelties .........................................................................................8 3.4 Other Devices ................................................................................................9 3.6 Specific Requirements for Consumer Fireworks...........................................10 3.7 Prohibited Chemicals and Components.........................................................12 3.8 Requirements for Theatrical Pyrotechnics ....................................................13 3.9 Approval ........................................................................................................13 3.10 Marking and Labeling..................................................................................14 4. REQUIREMENTS FOR DISPLAY FIREWORKS DEVICES ..........................14 4.1 Types of Display Fireworks Devices.............................................................14 4.2 Construction of Aerial Shells.........................................................................15 4.3 Approval -

Study of Ice-Forming Properties of Zinc Oxide and Silver Iodide Nanoreagents
Advances in Engineering Research, volume 177 International Symposium on Engineering and Earth Sciences (ISEES 2018) Study of Ice-Forming Properties of Zinc Oxide and Silver Iodide Nanoreagents Khuchunaev B.M. Kupovykh G.V. Department of Cloud Physics Institute of Computer Technologies and Information High-Mountain Geophysical Institute Security, Head of Department of Higher Mathematics, Nalchik, Russia Southern Federal University, Taganrog, Russia [email protected] [email protected] Teunova N.V. Khuchunaev B.M. Department of Cloud Physics Department of Cloud Physics High-Mountain Geophysical Institute High-Mountain Geophysical Institute Nalchik, Russia Nalchik, Russia [email protected] [email protected] Bekkiev M.Yu. Tashilova A.A. Director High-Mountain Geophysical Institute Department of Cloud Physics Nalchik, Russia High-Mountain Geophysical Institute [email protected] Nalchik, Russia [email protected] Panaetov V.P. Budaev A.Kh. Department of Cloud Physics Department of Cloud Physics High-Mountain Geophysical Institute High-Mountain Geophysical Institute Nalchik, Russia Nalchik, Russia [email protected] [email protected] Abstract—The article is devoted to the development of methods particles, which has a significant influence on phase transitions for dispersing nanoreagents based on zinc oxide and silver iodide of water and coagulation of reagent particles. and the study of their ice-forming properties. It is shown that nanotubes of zinc oxide and silver iodide have good ice-forming The modern period in the world of science is described by properties with a specific yield of ice-forming nuclei up to 1013г- the intensive development of experimental and theoretical 1. Unlike traditional reagents, the activity of zinc oxide nanotubes studies of the formation of a new phase in the nanoscale range. -

A Great File on Pyro-Losive
A GREAT FILE ON PYRO-LOSIVE VERY SIMPLIFIED AND INFORMATIVE A MUST READ The Explosives and Weapons Forum > Chemistry Discourse > Pyrotechnics > The KGB FIles http://www.roguesci.org/theforum/showthread.php?t=541 PDA View Full Version : The KGB FIles Boris October 2nd, 2002, 07:23 PM This has a bunch of black powder and ball mill files...They are all true,tried,and tested.....Most are written by me but if they are not I have permission from the author to post it....I am no where near done.I will have about 7-8 chapters when I am done and the high explosives chapter will have over 15 lessons...here is what I have so far...tell me if you see any mistakes.... The KGB Files By Boris Disclaimer: I hate disclaimers so I am not going to have one….even if I did write one you wouldn’t read it… just don’t fuck up. Chapter 1 Introductory and Equipment Lesson 1 A Word on Kewls Lesson 2 Lab Set-Up & Safety Lesson 3 Common Chemicals Lesson 4 Incompatible Chemicals Lesson 5 Glossary of Explosives Lesson 6 How to Make Your Own Ball Mill Lesson 7 Getting to Know Your Chemicals Chapter 2 Basic Introduction into Explosives and Chemistry Lesson 1 Chemical Formulas Lesson 2 Oxidation-Reduction Reactions Made Simple Lesson 3 Balanced Equations, or Stoichiometry Lesson 4 Converting Balanced Equations into Percentage Ratios Lesson 5 Combustion, Deflagration, and Detonation 2 Chapter 3 Low Explosives Lesson 1 Definition of Low Explosives Lesson 2 Safety for Low Explosives Lesson 3 Flash Powder Safety Lesson 4 Flash Powder Compositions Lesson 5 Black Powder Lesson 6 Thermite Lesson 2 Lab Set-Up & Safety By The Real This was written mostly by The Real. -

Un/Scetdg/29/Inf.33
UN/SCETDG/29/INF.33 COMMITTEE OF EXPERTS ON THE TRANSPORT OF DANGEROUS GOODS AND ON THE GLOBALLY HARMONIZED SYSTEM OF CLASSIFICATION AND LABELLING OF CHEMICALS Sub-Committee of Experts on the Transport of Dangerous Goods Twenty-ninth session Geneva, 3-12 (a.m.) July 2006 Item 2 of the provisional agenda TRANSPORT OF EXPLOSIVES Note 2 to 2.1.3.5.5 Submitted by the expert from the United Kingdom Introduction The expert from the United Kingdom has noticed a recent change in pyrotechnic compositions in fireworks manufactured in Asia. Previously, fireworks which produced report effects were manufactured using flash compositions comprising an oxidising substance (e.g. potassium perchlorate or barium nitrate) and a metal fuel (e.g. aluminium). Recent firework classification applications have shown a trend to compositions which do not meet the definition in Note 2 to 2.1.5.2.2 since the metal powder element has been replaced by energetic organic compounds such benzoates (Whistle powder), or metal sulfides such as antimony sulfide. Mixtures of potassium perchlorate/benzoates or potassium perchlorate/antimony sulfides have an explosive power similar to, or exceeding that of, traditional barium nitrate/aluminium flash compositions. The accidents at Enschede, Kolding were, in part, due to the presence of fireworks containing a large proportion of oxidiser/metal flash composition. The recent explosion aboard the Hyundai Fortune off the Dubai coast was reported to have been involved fireworks. Discussion Note 2 to Paragraph 2.1.3.5.5 defines flash composition as being “Flash composition” in this table refers to pyrotechnic compositions containing an oxidizing substance, or black powder, and a metal powder fuel that are used to produce an aural report effect or used as a bursting charge in fireworks devices.” Firework articles containing perchlorate/benzoate pyrotechnic composition would not be considered to be flash compositions but could produce explosion effects similar, or greater than, traditional flash compositions. -

APA 87-1: Standard for Construction and Approval for Transportation Of
By Authority Of THE UNITED STATES OF AMERICA Legally Binding Document By the Authority Vested By Part 5 of the United States Code § 552(a) and Part 1 of the Code of Regulations § 51 the attached document has been duly INCORPORATED BY REFERENCE and shall be considered legally binding upon all citizens and residents of the United States of America. HEED THIS NOTICE: Criminal penalties may apply for noncompliance. e Document Name: CFR Section(s): Standards Body: Official Incorporator: THE EXECUTIVE DIRECTOR OFFICE OF THE FEDERAL REGISTER WASHINGTON, D.C. APA STANDARD 87-1 Contents 1. INTRODUCTION ............................................................................................. 1 2. DEFINITIONS .................................................................................................... 1 3. REQUIREMENTS FOR CONSUMER FIREWORKS, NOVELTIES AND THEATRICAL pyROTECHNICS ..................................................................... 4 3.1 Types of Consumer Fireworks ...................................................................... 5 3.2 Types of Novelties ........................................................................................ 8 3.4 Other Devices ................................................................................................ 9 3.6 Specific Requirements for Consumer Fireworks .......................................... 10 3.7 Prohibited Chemicals and Components ........................................................ 12 3.8 Requirements for Theatrical Pyrotechnics .................................................. -

Pyrotechnic Ignition and Propagation: a Review
An earlier draft appeared in Journal of Pyrotechnics, No. 6, 1997. Pyrotechnic Ignition and Propagation: A Review K. L. and B. J. Kosanke PyroLabs, Inc., 1775 Blair Road, Whitewater, CO 81527, USA ABSTRACT chemical energy unless externally stimulated in some way. Probably the most common stimulus is The ideal pyrotechnic is completely stable in the addition of heat, such as provided by a burning storage and handling, yet performs its mission match or fuse. Ignition is the process of stimulat- completely, with absolute reliability, upon de- ing a pyrotechnic composition to release its inter- mand. Many accidents in pyrotechnics are the nal energy and can be defined as “the initiation of result of unintentional ignitions during handling self-sustained burning or explosion of a pyrotech- and storage. There can also be serious safety nic material”.[3] ramifications of ignition and propagation failures of pyrotechnic devices. This review article pre- Figure 1 illustrates the process of ignition by sents a fairly rigorous, but mostly non- graphing the internal energy of a tiny portion of mathematical discussion of the ignition and prop- pyrotechnic composition during the progress of its agation processes. chemical reactions and is typical of non- spontaneous exothermic chemical reactions.[4] At the left of the graph, where the process begins, the Keywords: ignition, propagation, heat of reaction, pyrotechnic composition has a certain amount of activation energy, spontaneous ignition, thermal internal energy. To accomplish ignition, external run-away, cook-off energy is supplied, such as from a burning match. This addition of energy increases the internal en- ergy of the composition and is seen as a rise in the Introduction curve of Figure 1.